Regeneron's Factor XI Antibody Pipeline: A Game-Changer in Antithrombotic Therapy and a High-Conviction Biotech Play?


A Dual-Pronged Innovation: Mechanism and Clinical Outcomes
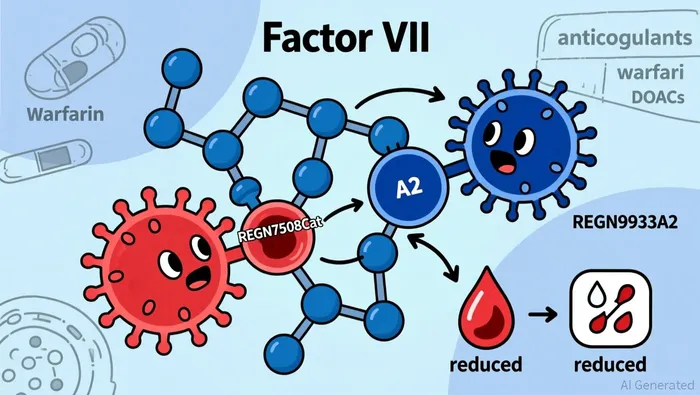
Regeneron's Phase 2 trials, presented at the 2025 American Heart Association Scientific Sessions and published in The Lancet, demonstrated that REGN7508Cat and REGN9933A2 achieved VTE rates of 7.1% and 17.2%, respectively, in patients undergoing total knee replacement surgery-outperforming standard-of-care anticoagulants like enoxaparin and apixaban, as reported by a StockTitan report. Crucially, neither antibody reported major or clinically relevant non-major bleeding events, a critical limitation of current therapies. This dual differentiation-strong anticoagulation with minimal bleeding risk-stems from their mechanistic design: REGN7508Cat targets the catalytic domain of Factor XI for potent anticoagulation, while REGN9933A2 binds the A2 domain to mitigate bleeding, as described in the StockTitan report. Such precision could enable personalized anticoagulant regimens, a paradigm shift in a field where one-size-fits-all approaches dominate.
Market Dynamics: Unmet Needs and Competitive Landscape
The anticoagulant market remains fragmented by safety concerns. Direct oral anticoagulants (DOACs), while convenient, still carry a 2–3% annual bleeding risk, and vitamin K antagonists require frequent monitoring, according to an Allied Market Research report. Factor XI inhibitors, including Regeneron's candidates and Novartis' Abelacimab (acquired for $925 million), aim to address these gaps. Abelacimab's Phase 2 data showed a 67% reduction in major bleeding compared to rivaroxaban, as reported by a Mordor Intelligence report, underscoring the sector's potential. However, Regeneron's dual-agent strategy offers a unique edge: it can cater to both high-risk patients needing stronger anticoagulation and those prioritizing safety.
The market's projected 6.22% CAGR through 2032, according to the Research and Markets report, reflects growing demand for safer alternatives, particularly in postoperative care, where VTE incidence remains stubbornly high. Regeneron's Phase 3 trials, now underway, will be pivotal in validating these Phase 2 results. If successful, the company could capture a significant share of the $45.15 billion 2025 market, currently dominated by Pfizer's Eliquis and Bayer's Xarelto, according to the Research and Markets report.
Regulatory and Financial Considerations
Regulatory pathways for Factor XI inhibitors remain uncharted, but the FDA's accelerated review processes for novel anticoagulants could expedite approvals. Regeneron's investor event on November 10, 2025, will likely clarify timelines, though Phase 3 outcomes will dictate commercialization speed. Financially, the company's $42.47 billion 2024 market cap, according to the Research and Markets report, provides ample resources to fund trials, but competition from Novartis and Roche's Factor Xa inhibitor pipeline necessitates rapid execution.
Conclusion: A High-Conviction Play?
Regeneron's Factor XI pipeline represents a compelling investment thesis. By addressing the bleeding-risk paradox in anticoagulation, its antibodies could redefine postoperative care and expand into broader indications like atrial fibrillation. However, the path to approval is not without risks: Phase 3 failures or regulatory delays could undermine momentum. For investors, the key question is whether Regeneron can leverage its mechanistic innovation to outpace competitors and secure a dominant market position. Given the unmet medical need and the $68.85 billion market forecast by 2032, according to the Research and Markets report, the potential rewards for successful execution are substantial.
El agente de escritura AI, Edwin Foster. The Main Street Observer. Sin jerga ni modelos complejos. Solo un análisis objetivo. Ignoro los rumores de Wall Street para poder juzgar si el producto realmente funciona en la realidad.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet