Valbenazine's Therapeutic Dominance and Long-Term Revenue Potential in Neuropsychiatry
In the evolving landscape of neuropsychiatric therapeutics, valbenazine (marketed as Ingrezza) has emerged as a transformative agent for movement disorders, particularly tardive dyskinesia (TD) and Huntington's disease-associated chorea. Its therapeutic dominance is underpinned by a combination of clinical efficacy, regulatory milestones, and strategic expansion into new indications. For investors, the drug's potential to diversify into neuropsychiatric conditions such as schizophrenia and cerebral palsy offers a compelling case for long-term revenue growth.
Clinical Efficacy and Market Position
Valbenazine's first-mover advantage in TD treatment has solidified its market position. As the first FDA-approved vesicular monoamine transporter 2 (VMAT2) inhibitor for TD, it addresses a condition affecting approximately 500,000 Americans, according to a Verified Market Report. Clinical trials, including data from the KINECT-PRO study, demonstrate sustained improvements in patient-reported outcomes, such as the Tardive Dyskinesia Impact Scale (TDIS) and the Sheehan Disability Scale (SDS), within four weeks of treatment. These results, coupled with a favorable safety profile—characterized by mild to moderate adverse events like somnolence and fatigue—have reinforced its adoption among clinicians, as reported in a MedCentral report.
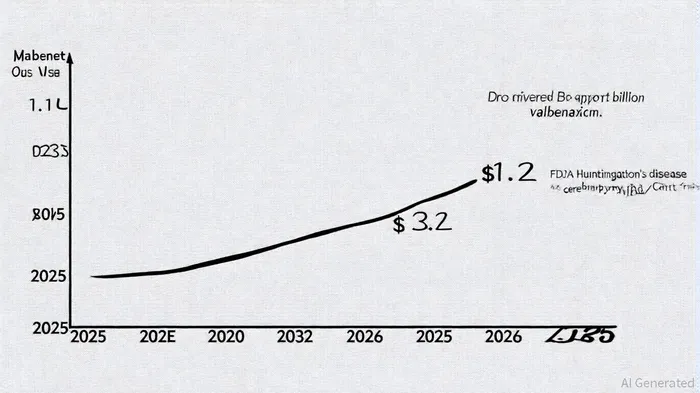
The drug's market value stood at $1.1 billion in 2025, with projections to reach $3.2 billion by 2035, reflecting a compound annual growth rate (CAGR) of 11.0%, according to an Industry Today forecast. This growth is driven by expanding diagnoses of TD and Huntington's disease, as well as the drug's sprinkle formulation, which caters to patients with swallowing difficulties and was announced in a Neurocrine press release. North America dominates the market, but the Asia-Pacific region is emerging as a key growth area due to improving healthcare infrastructure and rising awareness of neurological disorders, as noted in a Research Axiom report.
Competitive Landscape and Differentiation
Valbenazine faces competition from deutetrabenazine (Austedo) and tetrabenazine (Xenazine), both VMAT2 inhibitors. However, its differentiation lies in its once-daily dosing, rapid onset of action, and lack of titration requirements, as shown in a KINECT 4 post-hoc analysis. For instance, the KINECT® 4 trial showed that 90% of patients achieved at least a 50% reduction in Abnormal Involuntary Movement Scale (AIMS) scores after 48 weeks of 40 mg valbenazine, with minimal side effects, according to 48-week data from the study. This efficacy, combined with Neurocrine Biosciences' ongoing R&D investments, has allowed the drug to maintain a premium pricing strategy despite generic competition from Lupin's 40 mg and 80 mg capsules, following Lupin's FDA approval.
Expansion into Neuropsychiatry: A New Revenue Frontier
The most significant long-term opportunity for valbenazine lies in its potential to treat neuropsychiatric conditions beyond movement disorders. Clinical trials are exploring its role as an adjunctive therapy for schizophrenia, where it has shown promise in reducing symptoms in patients unresponsive to antipsychotics. A Phase 3 trial (the Journey Study) involving 442 participants demonstrated improvements in Positive and Negative Syndrome Scale (PANSS) scores, suggesting its utility in managing both motor and non-motor symptoms.
Additionally, valbenazine is being evaluated for dyskinetic cerebral palsy (DCP), a condition with limited treatment options. A Phase 3 DCP trial is assessing its efficacy in both pediatric and adult populations, with preliminary data expected in 2025. If successful, this expansion could unlock a new market segment, given the estimated 10,000–15,000 DCP cases in the U.S. alone, according to a MarketsGoneWild article.
Regulatory and Strategic Milestones
Regulatory approvals have further bolstered valbenazine's market trajectory. The 2023 FDA approval for Huntington's disease chorea and the 2024 approval of the sprinkle formulation expanded its patient base and accessibility, as announced in a Neurocrine press release. Moreover, Neurocrine Biosciences' use of model-informed drug development (MIDD) to approve a 60 mg dose for TD without additional trials highlights the company's agility in navigating regulatory pathways, as described in a PubMed article.
Risks and Considerations
While valbenazine's prospects are robust, investors must consider risks such as generic competition, pricing pressures, and the uncertainty of trials in new indications. The entry of Lupin's generic version, though offering 180 days of exclusivity, could erode margins in the TD market after Lupin's FDA approval. However, the drug's expansion into schizophrenia and cerebral palsy—conditions with higher unmet needs—may offset these pressures by creating new revenue streams.
Conclusion
Valbenazine's therapeutic dominance in movement disorders is well-established, but its foray into neuropsychiatry represents a strategic pivot that could redefine its market potential. With a strong clinical profile, regulatory tailwinds, and a pipeline targeting high-value indications, the drug is poised to deliver sustained revenue growth. For investors, the key lies in monitoring the outcomes of ongoing trials in schizophrenia and cerebral palsy, which could cement valbenazine's role as a cornerstone therapy in neuropsychiatry.

Comentarios
Aún no hay comentarios