RYBREVANT + LAZCLUZE: A Paradigm Shift in First-Line EGFR-Mutated Lung Cancer Treatment
The oncology landscape is undergoing a seismic shift as Johnson & Johnson’s RYBREVANT (amivantamab) in combination with LAZCLUZE (lazertinib) redefines the standard of care for first-line treatment of EGFR-mutated non-small cell lung cancer (NSCLC). This chemotherapy-free regimen, approved in the U.S., EU, and Canada, has demonstrated unprecedented clinical outcomes in the Phase 3 MARIPOSA trial, offering a compelling case for its long-term value and disruptive potential in a market dominated by AstraZeneca’s osimertinib (Tagrisso).
Clinical Efficacy: A New Benchmark in Survival Outcomes
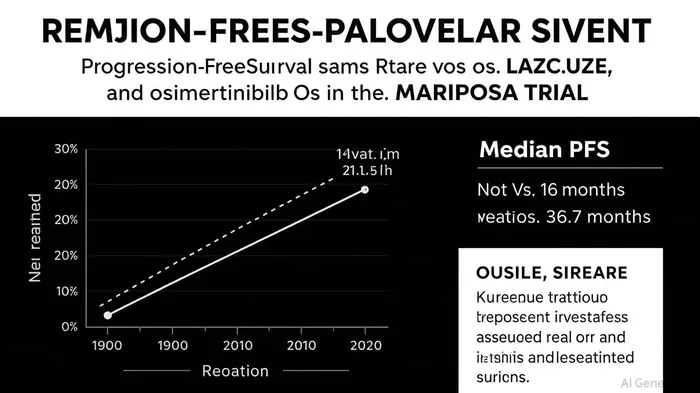
According to a report by Johnson & Johnson, the MARIPOSA trial revealed that RYBREVANT+LAZCLUZE extended median progression-free survival (PFS) to 23.7 months, compared to 16.6 months with osimertinib [1]. More strikingly, median overall survival (OS) for the combination therapy has not yet been reached, while osimertinib’s median OS stood at 36.7 months at the time of analysis [1]. At three years, 61% of patients on RYBREVANT+LAZCLUZE remained alive, versus 53% on osimertinib [2]. These results are not merely statistically significant but clinically transformative, particularly given the regimen’s ability to reduce resistance mechanisms. MET amplifications occurred in just 3% of patients on the combination versus 13% with osimertinib, while secondary EGFR mutations were observed in 1% versus 8% [1].
Regulatory Momentum and Market Access
The rapid regulatory approvals of RYBREVANT+LAZCLUZE underscore its therapeutic promise. Health Canada authorized the combination in March 2025 for EGFR exon 19 deletion or L858R mutation-positive NSCLC [3]. The U.S. FDA granted approval in August 2024, citing a “clinically meaningful improvement in overall survival” [3]. The European Commission followed suit in January 2025, with the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommending the therapy in November 2024 [4]. These approvals, supported by robust clinical data, position RYBREVANT+LAZCLUZE as a chemotherapy-free alternative in a market where prior options often required cytotoxic agents.
Market Disruption: Challenging Osimertinib’s Dominance
Osimertinib has long been the gold standard in EGFR-mutated NSCLC, generating nearly $6.6 billion in annual sales [5]. However, RYBREVANT+LAZCLUZE’s superior survival outcomes and resistance profile are reshaping competitive dynamics. GlobalData forecasts combined sales of $4.1 billion for the Janssen regimen by 2030, reflecting its potential to capture a significant share of the $300 million EGFR-mutated NSCLC market [5]. This growth is further fueled by the global lung cancer market’s projected 12.6% CAGR, reaching $64.53 billion by 2032 [6].
Yet, the path to widespread adoption is not without hurdles. Cost-effectiveness analyses highlight the higher acquisition costs of RYBREVANT and LAZCLUZE compared to osimertinib, which is often paired with generic chemotherapies [7]. For the combination to meet cost-effectiveness thresholds of $50,000 per quality-adjusted life year (QALY), price reductions or innovative reimbursement models may be necessary [7]. Nevertheless, the regimen’s ability to delay resistance and reduce hospitalizations for adverse events could offset upfront costs over time.
Strategic Implications for Investors
RYBREVANT+LAZCLUZE represents more than a product launch; it is a strategic pivot for Janssen in the oncology space. By addressing unmet needs in EGFR-mutated NSCLC—namely, resistance development and chemotherapy dependency—the combination therapy strengthens Janssen’s portfolio against competitors. For investors, the key risks lie in pricing pressures and reimbursement challenges, while the rewards hinge on the therapy’s ability to sustain its clinical edge and secure favorable formulary placement.
Conclusion
The MARIPOSA trial’s results have established RYBREVANT+LAZCLUZE as a paradigm shift in EGFR-mutated NSCLC treatment. With regulatory approvals, clinical superiority, and a growing market, this chemotherapy-free regimen is poised to disrupt a $64 billion industry. However, its long-term success will depend on navigating cost-effectiveness debates and maintaining its lead in an increasingly competitive therapeutic landscape. For now, the data speaks volumes: in the race to extend life and improve quality of care, Janssen has set a new benchmark.
Source:
[1] RYBREVANT® (amivantamab-vmjw) plus LAZCLUZE™ (lazertinib) Significantly Outperforms Standard of Care in First-Line EGFR-Mutated Lung Cancer with Compelling New Data at ELCC 2025 [https://www.prnewswire.com/news-releases/rybrevant-amivantamab-vmjw-plus-lazcluze-lazertinib-significantly-outperforms-standard-of-care-in-first-line-egfr-mutated-lung-cancer-with-compelling-new-data-at-elcc-2025-302406501.html]
[2] RYBREVANT® (amivantamab-vmjw) plus LAZCLUZE® (lazertinib) prevents acquired resistance versus osimertinib in first-line EGFR-mutated non-small cell lung cancer [https://www.prnewswire.com/news-releases/rybrevant-amivantamab-vmjw-plus-lazcluze-lazertinib-prevents-acquired-resistance-versus-osimertinib-in-first-line-egfr-mutated-non-small-cell-lung-cancer-302548296.html]
[3] Health Canada Approves Amivantamab/Lazertinib Combo for EGFR-Advanced NSCLC [https://www.onclive.com/view/health-canada-approves-amivantamab-lazertinib-combo-for-egfr-advanced-nsclc]
[4] European Commission approves LAZCLUZE® (lazertinib) in combination with RYBREVANT® (amivantamab) for the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer [https://www.jnj.com/media-center/press-releases/european-commission-approves-lazcluze-lazertinib-in-combination-with-rybrevant-amivantamab-for-the-first-line-treatment-of-patients-with-egfr-mutated-advanced-non-small-cell-lung-cancer]
[5] J&J's Lazcluze/Rybrevant combination obtains European approval to treat NSCLC [https://www.pharmaceutical-technology.com/news/jjs-lazcluze-rybrevant-combination-obtains-european-approval-to-treat-nsclc/]
[6] Lung Cancer Market Size & Opportunity 2025-2032 [https://www.coherentmarketinsights.com/industry-reports/lung-cancer-market]
[7] Dr Lopes on a Cost Analysis of Osimertinib/Chemo vs Amivantamab/Lazertinib in EGFR-mutated NSCLC [https://www.onclive.com/view/dr-lopes-on-a-cost-analysis-of-osimertinib-chemo-vs-amivantamab-lazertinib-in-egfr-nsclc]

Comentarios
Aún no hay comentarios