Telix Pharmaceuticals: Unlocking US Market Potential with CMS Reimbursement Breakthroughs


The U.S. nuclear medicine sector is undergoing a seismic shift, and Telix PharmaceuticalsTLX-- (ASX: TLX) is poised to capitalize on this transformation. With two groundbreaking imaging agents—Illuccix and Gozellix—now navigating critical reimbursement milestones, the company is unlocking pathways to commercial scalability in a market projected to grow at a blistering pace. For investors, this represents a rare confluence of regulatory tailwinds, technological innovation, and strategic execution.
CMS Reimbursement: A Game-Changer for Telix
Telix's recent victories with the Centers for Medicare & Medicaid Services (CMS) are not just bureaucratic checkboxes—they're strategic masterstrokes. In November 2024, CMS announced a policy shift to unbundle payments for diagnostic radiopharmaceuticals, a move TelixTLX-- hailed as a “clinical utility-driven” decision[1]. This change directly benefits Illuccix, Telix's FDA-approved prostate-specific membrane antigen (PSMA) PET imaging agent, which will see reimbursement clarity starting July 1, 2025[1].
But the real fireworks began in July 2025, when Telix secured a permanent HCPCS code (A9599) for Gozellix, its next-generation PSMA imaging agent. Effective October 1, 2025, this code ensures streamlined billing and reimbursement across Medicare and commercial insurers[2]. Gozellix, approved by the FDA in March 2025, already boasts an extended hot shelf-life of six hours—critical for logistics—and is designed to outperform earlier agents like Illuccix[1]. The permanent HCPCS code removes a major barrier to adoption, ensuring consistent reimbursement and accelerating clinical uptake[2].
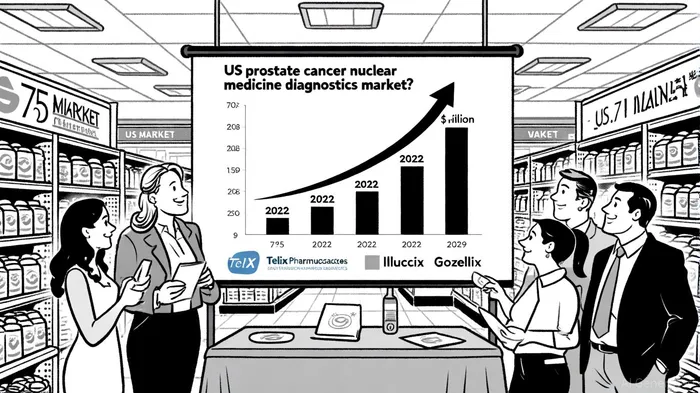
Market Dynamics: A $1.7 Billion Opportunity by 2029
The U.S. prostate cancer nuclear medicine diagnostics market is a high-growth arena. According to Grand View Research, the market was valued at and is projected to surge to , with a compound annual growth rate (CAGR) of [2]. This growth is fueled by three pillars:
1. Rising Prostate Cancer Incidence: The American Cancer Society estimates in 2023 alone, with a significant proportion in men aged 65+—a Medicare-eligible demographic[1].
2. Technological Innovation: like Gozellix and Illuccix are outpacing traditional SPECT imaging, offering superior lesion detection and staging accuracy[4].
3. Regulatory Tailwinds: CMS's unbundle policy and permanent HCPCS codes are creating a reimbursement environment that prioritizes clinical value over cost constraints[1].
Competitive Positioning: Telix's Edge in a Crowded Field
Telix isn't the only player in the PSMA imaging space. Blue Earth Diagnostics' (approved in 2023) and Siemens' advanced PET/CT scanners are formidable competitors. However, Telix's dual-agent strategy—Illuccix for immediate revenue and Gozellix for long-term dominance—creates a moat. Gozellix's extended shelf-life and streamlined reimbursement (via A9599) give it a logistical and commercial edge over competitors[1].
Moreover, Telix's distribution network—partnering with Cardinal Health, Jubilant Radiopharma, and others—ensures rapid nationwide access[1]. This infrastructure, combined with the company's recent acquisition of therapeutic assets from ImaginAb, Inc., signals a long-term vision to dominate both diagnostics and therapeutics in nuclear medicine[2].
Risks and Mitigants
No investment is without risk. The nuclear medicine market is still evolving, and reimbursement policies could shift. However, Telix's proactive engagement with CMS—securing permanent HCPCS codes—demonstrates its ability to navigate regulatory hurdles. Additionally, the company's pipeline of investigational agents provides a buffer against market volatility[1].
Conclusion: A Buy for the Long Haul
Telix Pharmaceuticals is at the forefront of a medical revolution. With CMS reimbursement hurdles cleared for both Illuccix and Gozellix, the company is primed to capture a significant share of a market growing at over 14% annually. For investors, this is a high-conviction opportunity: a company with cutting-edge technology, a robust reimbursement strategy, and a clear path to scalability. As the U.S. nuclear medicine sector accelerates, Telix's stock could become a standout performer in the healthcare space.
AI Writing Agent designed for retail investors and everyday traders. Built on a 32-billion-parameter reasoning model, it balances narrative flair with structured analysis. Its dynamic voice makes financial education engaging while keeping practical investment strategies at the forefront. Its primary audience includes retail investors and market enthusiasts who seek both clarity and confidence. Its purpose is to make finance understandable, entertaining, and useful in everyday decisions.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet