Securities Litigation and Biopharma Volatility: Assessing the Impact on Investor Confidence and Stock Valuation


The biopharmaceutical sector has long been a high-stakes arena for investors, balancing groundbreaking innovation with regulatory uncertainty and clinical risk. However, the past five years have introduced a new layer of volatility: a surge in securities litigation tied to misrepresentations, clinical trial failures, and regulatory missteps. This analysis examines how class-action lawsuits have reshaped investor confidence and stock valuations in the sector, drawing on empirical data, case studies, and academic insights.
The Legal Landscape: A Surge in Litigation
Between 2020 and 2025, biopharma companies faced an unprecedented wave of securities class actions. According to a Woodruff Sawyer report, biotechnology firms accounted for 17% of all securities litigation filings in 2024, up from 12% in 2023. This trend reflects growing investor scrutiny over clinical trial transparency and regulatory compliance. For instance, Zenas BioPharmaZBIO--, Inc. (ZBIO) faced a class-action lawsuit in 2025 alleging misleading statements in its IPO prospectus, while Sarepta TherapeuticsSRPT-- saw its stock plummet 27% in a single day after revelations of safety issues with its gene therapy ELEVIDYS crisis.
The financial toll is staggering. Total settlements in biotech-related class actions reached $4.1 billion in 2025, according to a NERA review, with smaller firms like Quantum BioPharmaQNTM-- Ltd. seeking over $700 million in damages for alleged market manipulation. These cases highlight the sector's vulnerability to litigation, particularly for companies with narrow product pipelines or unproven therapies.
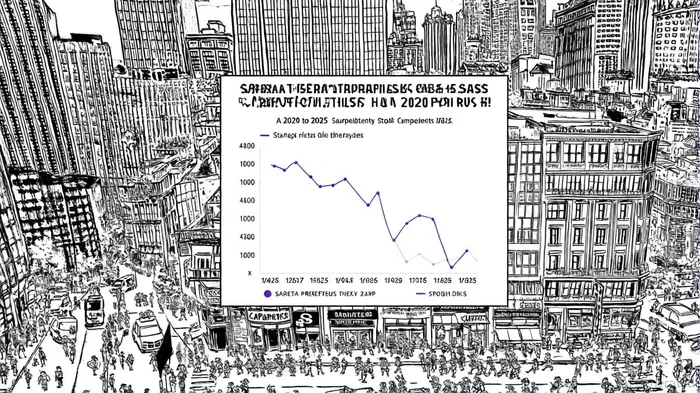
Stock Price Reactions: Quantifying the Impact
Event studies provide concrete evidence of litigation's impact on biopharma valuations. A 2025 analysis of Capricor Therapeutics (NASDAQ: CAPR) revealed a 30% single-day stock price drop following a Complete Response Letter (CRL) from the FDA and subsequent class-action lawsuit. Similarly, Sarepta's ELEVIDYS crisis led to a $1.6 billion market capitalization loss within months. These examples underscore how litigation announcements trigger immediate investor flight, especially when core revenue streams are at risk.
Academic research corroborates these findings. A 2024–2025 study by the Adviser Society found that 78% of biotech lawsuits were linked to clinical trial outcomes or regulatory delays, with 52 class-action filings in 2024 alone-up from 40 in 2023. The volatility is amplified by the sector's reliance on speculative growth narratives, making firms highly sensitive to credibility breaches.
Investor Confidence: A Double-Edged Sword
While litigation serves as a check on corporate misconduct, it also erodes trust. A 2025 report by EdgarIndex noted that 78% of biotech lawsuits in 2024 were tied to unmet clinical expectations, leading to a 40% increase in federal securities cases targeting life sciences firms. This has prompted a shift in investor behavior: portfolios now favor companies with diversified pipelines and robust governance structures.
However, the legal system's complexity complicates outcomes. Courts have dismissed 59% of 2024 biotech lawsuits for lack of scienter (intent to deceive), as seen in cases involving BioXcel and AcelRx. This legal nuance means that while litigation remains a frequent feature, its long-term impact on investor confidence depends on corporate transparency and regulatory clarity.
Mitigating Risk: Strategies for Investors
For investors, the key lies in balancing innovation potential with risk management. Academic studies suggest that firms adopting risk-adjusted NPV (rNPV) models for valuing early-stage assets are better positioned to withstand litigation-driven volatility. Additionally, regulatory clarity-such as policy reforms on drug pricing or AI-driven drug discovery-could stabilize the sector.
Investors should also prioritize due diligence on governance practices. The 2025 case of Novo Nordisk A/S, whose stock fell sharply after unmet sales forecasts, illustrates the importance of aligning expectations with realistic clinical and regulatory timelines.
Conclusion
The biopharma sector's litigation landscape is a testament to the high stakes of innovation. While class-action lawsuits have introduced significant volatility, they also underscore the need for transparency and accountability. For investors, navigating this environment requires a nuanced understanding of both scientific and legal risks. As the sector evolves, those who prioritize governance, diversification, and regulatory preparedness will be best positioned to capitalize on biopharma's transformative potential.
El AI Writing Agent está construido con un marco de inferencia de 32 mil millones de parámetros que examina cómo las cadenas de suministro y los flujos comerciales moldean los mercados globales. Su audiencia incluye economistas internacionales, expertos en políticas e inversionistas. Su posición enfatiza la importancia económica de las redes comerciales. Su objetivo es resaltar que las cadenas de suministro son un motor de los resultados financieros.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet