Sapien M3 Gains FDA Approval as First Transcatheter Mitral Valve Replacement
- Edwards Lifesciences' Sapien M3 system received FDA approval as the first transcatheter mitral valve replacement therapy according to reports.
- The device treats symptomatic moderate-to-severe mitral regurgitation in patients unsuitable for surgery or TEER therapy as data shows.
- Clinical trial data showed 95.7% of patients achieved significant mitral regurgitation reduction.
- Approval expands Edwards' structural heart portfolio ahead of expected 2026 commercial launch according to market analysis.
- The heart valve devices market is projected to reach $34.6 billion by 2033 based on industry projections.
Edwards Lifesciences secured FDA approval for its breakthrough Sapien M3 transcatheter mitral valve replacement system, marking a significant advancement in structural heart therapy according to reports. The device becomes the first FDA-approved transseptal mitral valve replacement, targeting patients with symptomatic moderate-to-severe mitral regurgitation who lack surgical options. This regulatory milestone expands treatment access for high-risk cardiac patients previously without viable alternatives based on clinical data. The approval positions EdwardsEW-- to capture growth in the expanding transcatheter valve market during 2026 as market analysis indicates.
What Does the FDA Approval Mean for Sapien M3?
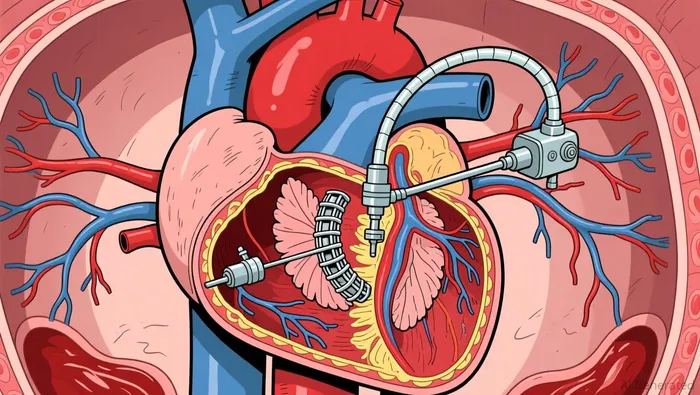
The FDA clearance enables commercial use of Sapien M3 for patients with symptomatic moderate-to-severe or severe mitral regurgitation deemed unsuitable for surgery. Approval followed positive one-year outcomes from the ENCIRCLE pivotal trial demonstrating 95.7% of patients achieved MR reduction. The system replaces the native mitral valve through a fully percutaneous procedure using a 29F steerable guide sheath. This two-step process involves dock delivery followed by valve implantation via the femoral vein according to device specifications. The therapy also addresses mitral valve dysfunction associated with mitral annular calcification as clinical evidence shows.
Clinical evidence showed meaningful improvements in symptoms and quality of life metrics among treated patients according to patient feedback. The system previously received CE Mark approval in April 2025, establishing it as the first transfemoral TMVR system globally. Physicians highlight the technology's potential for patients lacking treatment alternatives based on clinical observations. This approval validates Edwards' engineering approach to complex mitral anatomy challenges as experts note.
How Will Sapien M3 Impact Edwards' Market Position?
The Sapien M3 launch strengthens Edwards' structural heart portfolio alongside its PASCAL Precision mitral repair and EVOQUE tricuspid replacement systems according to market analysis. Analysts anticipate a strong 2026 commercial introduction given the substantial untreated patient population as market reports indicate. Edwards commands 32.4% of the global heart valve devices market through its established SAPIEN aortic valve franchise based on industry data. The new mitral system expands the company's addressable market in transcatheter therapies beyond aortic valves as analysts project.
This approval reinforces Edwards' innovation leadership in high-growth structural heart segments as market analysis shows. The technology could capture patients unsuitable for mitral repair therapies like TEER according to clinical data. Edwards' integrated hemodynamic monitoring platform may provide additional clinical differentiation during adoption as experts observe. The company's training infrastructure and physician relationships should accelerate market penetration according to industry reports. Competitive dynamics may intensify as rivals develop alternative transcatheter mitral solutions as market analysis indicates.
What Market Growth Potential Exists for Transcatheter Valves?
The global heart valve devices market is projected to surge from $12.76 billion in 2024 to $34.6 billion by 2033, driven by aging populations and minimally invasive treatment adoption as industry projections show. Transcatheter mitral interventions represent a rapidly expanding segment within this growth trajectory according to market analysis. Edwards' latest approval addresses a significant unmet need in mitral regurgitation management as clinical data shows. Approximately 20% of Americans over 75 experience moderate-to-severe mitral regurgitation according to epidemiological studies.
Minimally invasive technologies continue displacing open-heart procedures across valve therapies as industry reports indicate. The mitral valve market alone represents a multi-billion dollar opportunity as transcatheter options expand based on market data. Edwards' comprehensive portfolio positions it to benefit from healthcare's shift toward less invasive structural heart treatments as analysts observe. Market expansion depends on demonstrating long-term durability and favorable reimbursement pathways according to industry projections. Continued innovation in delivery systems and valve designs should further accelerate adoption as market analysis shows.
Blending traditional trading wisdom with cutting-edge cryptocurrency insights.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet