Revolutionizing Heart Failure Treatment: The SynCardia Total Artificial Heart and Picard Medical's Investment Potential
- The SynCardia Total Artificial Heart (TAH) is the world's first and only commercially approved device in the US that fully replaces a failing human heart according to AZBio , serving as a bridge to transplant for patients with end-stage biventricular heart failure.
- Picard Medical, Inc. (PMI), the parent company of SynCardia Systems, LLC, went public in 2025 and is pioneering next-generation innovations like the fully implantable Emperor TAH, which could expand the market by eliminating external drivers.
- With heart failure affecting millions globally, the artificial heart sector holds significant growth potential, driven by technological advancements and an aging population.
- Investing in PMI offers exposure to the medtech industry, but requires monitoring regulatory approvals, clinical trials, and market adoption, alongside risks like development delays and competition.
- Evergreen opportunities in this field include long-term bets on biotech innovations, with historical precedents showing high returns for early investors in life-saving devices.
The field of artificial hearts represents a pinnacle of medical innovation, blending engineering prowess with life-saving potential. At the forefront stands the SynCardia Total Artificial Heart (TAH), developed by SynCardia Systems, LLC, a subsidiary of Picard MedicalPMI--, Inc. This device has transformed treatment options for patients with severe heart failure, offering a mechanical bridge to transplantation when no other alternatives exist. In this evergreen exploration, we'll delve into the technology behind the SynCardia TAH, its evolution under Picard Medical, and the broader implications for investors eyeing the burgeoning cardiac device market.
A Brief History of Artificial Hearts and SynCardia's Role
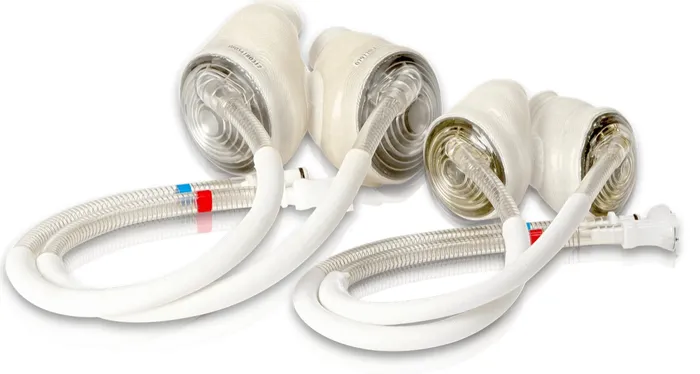
The quest for an artificial heart dates back to the mid-20th century, with early prototypes like the Jarvik-7 making headlines in the 1980s for their groundbreaking but limited success. SynCardia Systems, founded in 2001, built on this legacy by commercializing the temporary Total Artificial Heart (TAH-t), which received FDA approval in 2004 as a bridge-to-transplant device. Over the years, the company faced challenges, including a 2016 bankruptcy filing, after which it was acquired by Versa Capital Management. In a pivotal move, Picard Medical, Inc. became the holding company, fully owning SynCardia and steering it toward new innovations.
Today, the SynCardia TAH remains the only commercially available total artificial heart approved by the FDA and Health Canada, according to the official website , with over 2,100 implants worldwide. Its design mimics the natural heart's function, pumping blood to sustain life in patients awaiting a donor organ.
The Technology and Clinical Impact
According to National Institute of Health, The SynCardia TAH is a pneumatic device that completely replaces both ventricles of the heart, connected to an external driver that powers its pulsatile flow. It is designed for patients with biventricular failure—where both sides of the heart are compromised—and has proven effective in stabilizing individuals until a transplant becomes available. Unlike ventricular assist devices (VADs) that support only one side, the TAH eliminates the failing heart entirely, reducing risks like blood clots from damaged tissue.
Under Picard Medical's leadership, the focus has shifted to the Emperor TAH, a fully implantable version that promises greater patient mobility and quality of life by removing the need for external components. Recent in vivo implantations and presentations at conferences like ISMCS 2025 highlight its potential to expand the market. This advancement addresses limitations of the current model, positioning SynCardia as a leader in cardiac replacement therapy.
Heart failure affects over 6 million Americans alone, with the global market for cardiac devices projected to grow significantly due to aging populations and rising chronic diseases. The SynCardia TAH's track record—over 20 years post-FDA approval—underscores its reliability, with ongoing R&D enhancing its efficacy.
Development Potential and Investment Opportunities
Picard Medical's stewardship has injected fresh momentum into SynCardia, with investments funding the Emperor's development and clinical trials. As a publicly traded entity (PMI), the company offers investors direct exposure to this niche. The medtech sector, including artificial organs, is ripe for growth, with parallels to successful investments in devices like pacemakers or stents.
Entry points for investors include monitoring milestones like FDA submissions for the Emperor, which could drive stock appreciation. With a market cap influenced by recent IPO proceeds of around $17-19 million, PMI represents a speculative but high-reward play in biotech. Broader trends, such as increasing demand for organ replacements amid donor shortages, bolster long-term potential.
However, as with any medtech investment, patience is key—regulatory hurdles and clinical outcomes can sway valuations.
FAQs for Aspiring Investors and Enthusiasts

1. How does the SynCardia Total Artificial Heart work, and why is it a game-changer for heart failure treatment?
The SynCardia TAH is a mechanical pump that replaces the heart's ventricles, powered by an external driver to circulate blood throughout the body. It's revolutionary because it serves as a complete substitute for patients with both sides of the heart failing, providing a reliable bridge until a transplant is possible, unlike partial support devices that may not suffice in severe cases. For newcomers to medtech, this means it buys critical time, improving survival rates in a condition affecting millions.
2. What key factors should I consider before investing in a company like Picard Medical?
Before diving in, evaluate the company's pipeline (e.g., the Emperor TAH's progress), financial health via quarterly reports, and market trends like rising heart disease prevalence. Use resources like FDA and Medical Publications for basics on biotech valuation—start by setting up free accounts and tracking PMI's news feeds for informed decisions.
3. What risks should I watch for when investing in artificial heart companies like PMI?
Key risks include regulatory delays (e.g., FDA approvals), competition from rivals like Carmat, and high R&D costs leading to dilution. Market volatility in biotech can cause sharp drops—always assess financial health via reports from SynCardia official website. Diversify to mitigate.
4. How can I identify entry points for investing in medtech stocks during market fluctuations?
Watch for catalysts like positive clinical trial results or regulatory milestones, which often lead to price surges—use alerts on AInvest Newswire to notify you of PMI updates. For beginners, consider dollar-cost averaging during dips, but always review analyst ratings on sites like Reuters to avoid buying at peaks.
5. What portfolio strategies work well for including innovative biotech like PMI without overexposing myself?
Aim for a balanced approach: allocate 5-10% of your portfolio to high-growth biotech like PMI, paired with stable ETFs in healthcare. Track long-term trends via AInvest articles on organ tech, and rebalance quarterly— this hedges against volatility while capturing upside from breakthroughs like fully implantable hearts.
The AInvest News Editorial Team consists of experienced financial journalists and editors who oversee all published content. While our newsroom leverages advanced AI tools to assist in data gathering and draft generation, every article is reviewed, fact-checked, and approved by human editors to ensure accuracy, clarity, and transparency.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet