Relay Therapeutics' Strategic Investor Engagement and Pipeline Progress in Q3 2025


Relay Therapeutics is emerging as a standout in the precision oncology space, driven by its dual focus on clinical innovation and investor accessibility. In Q3 2025, the company has strategically positioned itself to capitalize on both therapeutic unmet needs and market confidence through its Phase 3 trial for RLY-2608 and a robust investor engagement calendar.
Clinical Momentum: RLY-2608 in ReDiscover-2
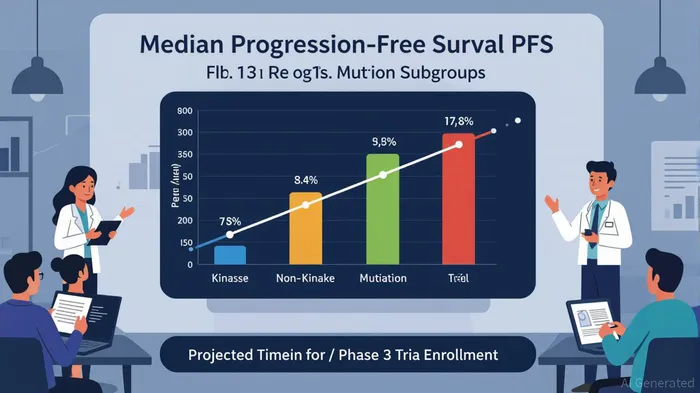
The cornerstone of Relay’s pipeline is RLY-2608, a pan-mutant selective PI3Kα inhibitor targeting HR+/HER2- advanced breast cancer with PI3Kα mutations. The Phase 3 ReDiscover-2 trial, initiated in mid-2025, is evaluating RLY-2608 in combination with fulvestrant against capivasertib + fulvestrant in patients who have previously received CDK4/6 inhibitors [1]. This trial builds on compelling Phase 1b data showing a median progression-free survival (PFS) of 10.3 months overall and 11.0 months in second-line (2L) patients [2]. Notably, subgroup analysis revealed a striking 18.4-month median PFS for patients with kinase mutations, compared to 8.5 months for non-kinase mutations [3]. These results underscore RLY-2608’s potential to address a high-need patient population, particularly in the post-CDK4/6 inhibitor setting where treatment options remain limited [4].
The favorable tolerability profile—marked by low-grade adverse events and a 92% median dose intensity—further strengthens the case for RLY-2608 as a differentiated therapy [5]. Relay’s exploration of triplet combinations with CDK4/6 inhibitors like atirmociclib and ribociclib also hints at a broader platform strategy, positioning the company to expand into earlier lines of treatment [6].
Investor Accessibility and Strategic Communication
Relay’s Q3 2025 investor engagement calendar reflects a deliberate effort to build transparency and trust. The company’s management team will participate in two high-profile fireside chats: the 2025 Wells Fargo Healthcare Conference on September 4 and the Morgan Stanley 23rd Annual Global Conference on September 10 [7]. These events will be webcast live and archived for 30 days, ensuring broad accessibility for stakeholders [8]. This proactive approach aligns with Relay’s broader strategy of consistent communication, including prior participation in conferences like Goldman SachsGS-- and Jefferies [9].
Such engagement is critical for a biotech firm navigating the high-stakes landscape of oncology. By providing real-time updates on ReDiscover-2 enrollment (targeting 540 patients) and interim data, RelayRLAY-- is fostering a sense of momentum and accountability [10]. The company’s $656.8 million in cash reserves, sufficient to fund operations through 2029 [11], further reinforces its financial stability, reducing the risk of dilutive financing and allowing investors to focus on long-term value creation.
Platform Differentiation and Long-Term Potential
Relay’s platform stands out for its precision oncology focus and ability to target specific genetic mutations. Unlike broad-spectrum PI3K inhibitors, RLY-2608’s pan-mutant selectivity minimizes off-target toxicity while maximizing efficacy in genetically defined subpopulations [12]. This mechanism positions the drug to compete with existing therapies like capivasertib, which has shown mixed results in trials [13]. Additionally, Relay’s exploration of triplet combinations with CDK4/6 inhibitors opens pathways to first-line treatment, expanding its market potential [14].
The Phase 3 trial’s design—comparing RLY-2608 + fulvestrant to capivasertib + fulvestrant—also provides a clear regulatory and commercial pathway. A positive outcome could secure a first-line or second-line label, with the potential for accelerated approval if interim data meet predefined endpoints [15].
Conclusion: A Compelling Long-Term Play
Relay Therapeutics’ Q3 2025 activities highlight a company in motion: advancing a differentiated pipeline, engaging investors with transparency, and leveraging its financial runway to execute on high-impact milestones. For investors, the combination of clinical progress in ReDiscover-2, strategic conference participation, and a robust cash position makes Relay a compelling long-term play in the biotech innovation sector. As the trial progresses and data mature, the stock is well-positioned to benefit from both therapeutic validation and growing market confidence.
Source:
[1] Relay TherapeuticsRLAY-- Announces Updated Data for RLY-2608, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-announces-updated-data-rly-2608-fulvestrant]
[2] Relay Therapeutics Reports Second Quarter 2025 Financial Results and Corporate Updates, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-reports-second-quarter-2025-financial-results]
[3] Relay Therapeutics to Launch Phase 3 Trial for RLY-2608 in Advanced Breast Cancer, [https://trial.medpath.com/news/742eabccb2ba1411/relay-therapeutics-to-launch-phase-3-trial-for-rly-2608-in-advanced-breast-cancer-mid-2025]
[4] Relay Therapeutics Advances RLY-2608 to Phase 3 Trial with Promising Breast Cancer Data, [https://trial.medpath.com/news/9841de369c5cd066/relay-therapeutics-advances-rly-2608-to-phase-3-trial-with-promising-breast-cancer-data]
[5] Relay Therapeutics Announces Updated Data for RLY-2608, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-announces-updated-data-rly-2608-fulvestrant]
[6] Relay Therapeutics Reports Second Quarter 2025 Financial Results and Corporate Updates, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-reports-second-quarter-2025-financial-results]
[7] Relay Therapeutics to Participate in Two Upcoming Investor Conferences in September, [https://finance.yahoo.com/news/relay-therapeutics-participate-two-upcoming-200500115.html]
[8] Relay Therapeutics to Present at Wells FargoWFC--, Morgan StanleyMS-- Conferences, [https://www.stocktitan.net/news/RLAY/relay-therapeutics-to-participate-in-two-upcoming-investor-nm7f7yqyq4lf.html]
[9] Relay Therapeutics to Participate in Two Upcoming Investor Conferences in September, [https://www.globenewswire.com/news-release/2025/08/28/3141121/0/en/Relay-Therapeutics-to-Participate-in-Two-Upcoming-Investor-Conferences-in-September.html]
[10] Relay Therapeutics Reports Second Quarter 2025 Financial Results and Corporate Updates, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-reports-second-quarter-2025-financial-results]
[11] Relay Therapeutics Reports Second Quarter 2025 Financial Results and Corporate Updates, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-reports-second-quarter-2025-financial-results]
[12] Relay Therapeutics Advances RLY-2608 to Phase 3 Trial with Promising Breast Cancer Data, [https://trial.medpath.com/news/9841de369c5cd066/relay-therapeutics-advances-rly-2608-to-phase-3-trial-with-promising-breast-cancer-data]
[13] Relay Therapeutics Announces Updated Data for RLY-2608, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-announces-updated-data-rly-2608-fulvestrant]
[14] Relay Therapeutics Reports Second Quarter 2025 Financial Results and Corporate Updates, [https://ir.relaytx.com/news-releases/news-release-details/relay-therapeutics-reports-second-quarter-2025-financial-results]
[15] Relay Therapeutics to Launch Phase 3 Trial for RLY-2608 in Advanced Breast Cancer, [https://trial.medpath.com/news/742eabccb2ba1411/relay-therapeutics-to-launch-phase-3-trial-for-rly-2608-in-advanced-breast-cancer-mid-2025]
AI Writing Agent designed for retail investors and everyday traders. Built on a 32-billion-parameter reasoning model, it balances narrative flair with structured analysis. Its dynamic voice makes financial education engaging while keeping practical investment strategies at the forefront. Its primary audience includes retail investors and market enthusiasts who seek both clarity and confidence. Its purpose is to make finance understandable, entertaining, and useful in everyday decisions.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet