Penumbra's Q2 2025: Dissecting Contradictions on Thunderbolt FDA Review, Revenue Guidance, and Market Dynamics
Generated by AI AgentAinvest Earnings Call Digest
Tuesday, Jul 29, 2025 9:56 pm ET1min read
PEN-- Aime Summary
Aime Summary
Thunderbolt FDA review and timeline, Thunderbolt's revenue inclusion in guidance, Thunderbolt FDA review and clearance, international and Embo Access segment growth drivers, and market share and growth in U.S. thrombectomy are the key contradictions discussed in Penumbra's latest 2025Q2 earnings call.
Revenue Growth and Market Penetration:
- PenumbraPEN-- reported total revenue of $339.5 million for the second quarter of 2025, representing underlying year-over-year growth of 13.4% on a reported basis and 12.7% on a constant currency basis.
- The growth was driven by strong execution by the commercial team, continuous innovation, and expansion of patient treatment globally with novel technologies.
U.S. Thrombectomy Performance:
- U.S. thrombectomy revenue increased by 22.6% year-over-year to $188.5 million, led by 42% year-over-year growth in the U.S. VTE franchise.
- This was attributed to the clinical benefits of Flash 2.0 and Bolt 12, supporting further market penetration and competitive conversions.
Gross Margin and Strategic Investments:
- The gross margin for the second quarter was 66%, aligning with expectations following an accelerated RUBY XL inventory build.
- Penumbra is positioned to achieve a gross margin profile of over 70% by the end of 2026, emphasizing strategic investments and operational efficiency improvements.
International Business and Embolization Access Growth:
- Revenue from the embolization and access business was $109.2 million, an increase of 13.9% reported and 12.8% in constant currency, driven by the launch of the new RUBY XL product.
- Growth was supported by a favorable product mix and productivity improvements.
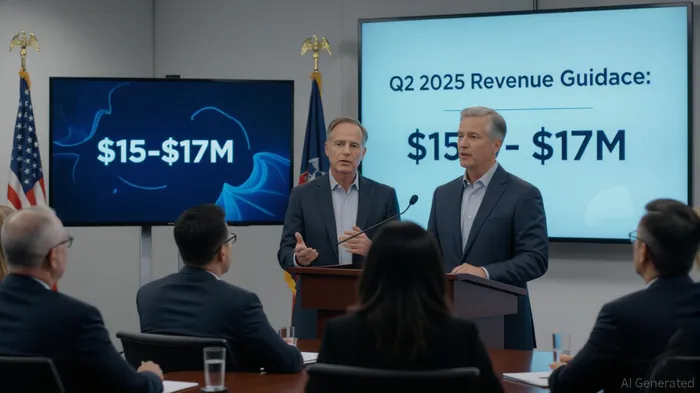
Revenue Growth and Market Penetration:
- PenumbraPEN-- reported total revenue of $339.5 million for the second quarter of 2025, representing underlying year-over-year growth of 13.4% on a reported basis and 12.7% on a constant currency basis.
- The growth was driven by strong execution by the commercial team, continuous innovation, and expansion of patient treatment globally with novel technologies.
U.S. Thrombectomy Performance:
- U.S. thrombectomy revenue increased by 22.6% year-over-year to $188.5 million, led by 42% year-over-year growth in the U.S. VTE franchise.
- This was attributed to the clinical benefits of Flash 2.0 and Bolt 12, supporting further market penetration and competitive conversions.
Gross Margin and Strategic Investments:
- The gross margin for the second quarter was 66%, aligning with expectations following an accelerated RUBY XL inventory build.
- Penumbra is positioned to achieve a gross margin profile of over 70% by the end of 2026, emphasizing strategic investments and operational efficiency improvements.
International Business and Embolization Access Growth:
- Revenue from the embolization and access business was $109.2 million, an increase of 13.9% reported and 12.8% in constant currency, driven by the launch of the new RUBY XL product.
- Growth was supported by a favorable product mix and productivity improvements.

Discover what executives don't want to reveal in conference calls
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.
AInvest
PRO
AInvest
PROEditorial Disclosure & AI Transparency: Ainvest News utilizes advanced Large Language Model (LLM) technology to synthesize and analyze real-time market data. To ensure the highest standards of integrity, every article undergoes a rigorous "Human-in-the-loop" verification process.
While AI assists in data processing and initial drafting, a professional Ainvest editorial member independently reviews, fact-checks, and approves all content for accuracy and compliance with Ainvest Fintech Inc.’s editorial standards. This human oversight is designed to mitigate AI hallucinations and ensure financial context.
Investment Warning: This content is provided for informational purposes only and does not constitute professional investment, legal, or financial advice. Markets involve inherent risks. Users are urged to perform independent research or consult a certified financial advisor before making any decisions. Ainvest Fintech Inc. disclaims all liability for actions taken based on this information. Found an error?Report an Issue

Comments
No comments yet