ONWARD Medical's ARC-IM Lumbar Lead: A New Hope for Spinal Cord Injury Patients
Generated by AI AgentMarcus Lee
Wednesday, Mar 26, 2025 2:42 am ET2min read
In the ever-evolving landscape of medical technology, ONWARD Medical N.V. has made a groundbreaking stride with the first human implant of its investigational ARC-IM® Lumbar Lead. This milestone, achieved on March 17, 2025, by Dr. Jocelyne Bloch at Lausanne University Hospital, marks a significant advancement in the field of spinal cord stimulation therapies. The ARC-IM Lumbar Lead is designed to target the lumbar region of the spinal cord, aiming to restore standing, stepping, and lower limb mobility in patients with spinal cord injuries (SCI).
The ARC-IM Lumbar Lead is the second model in the ARC-IM Lead family to be used in a clinical feasibility study. The company's portfolio of purpose-designed leads is part of a broader strategy to optimize the delivery of ARC-IM Therapy at various locations along the spinal cord. This approach is crucial for enabling the restoration of movement and other functions in SCI patients.
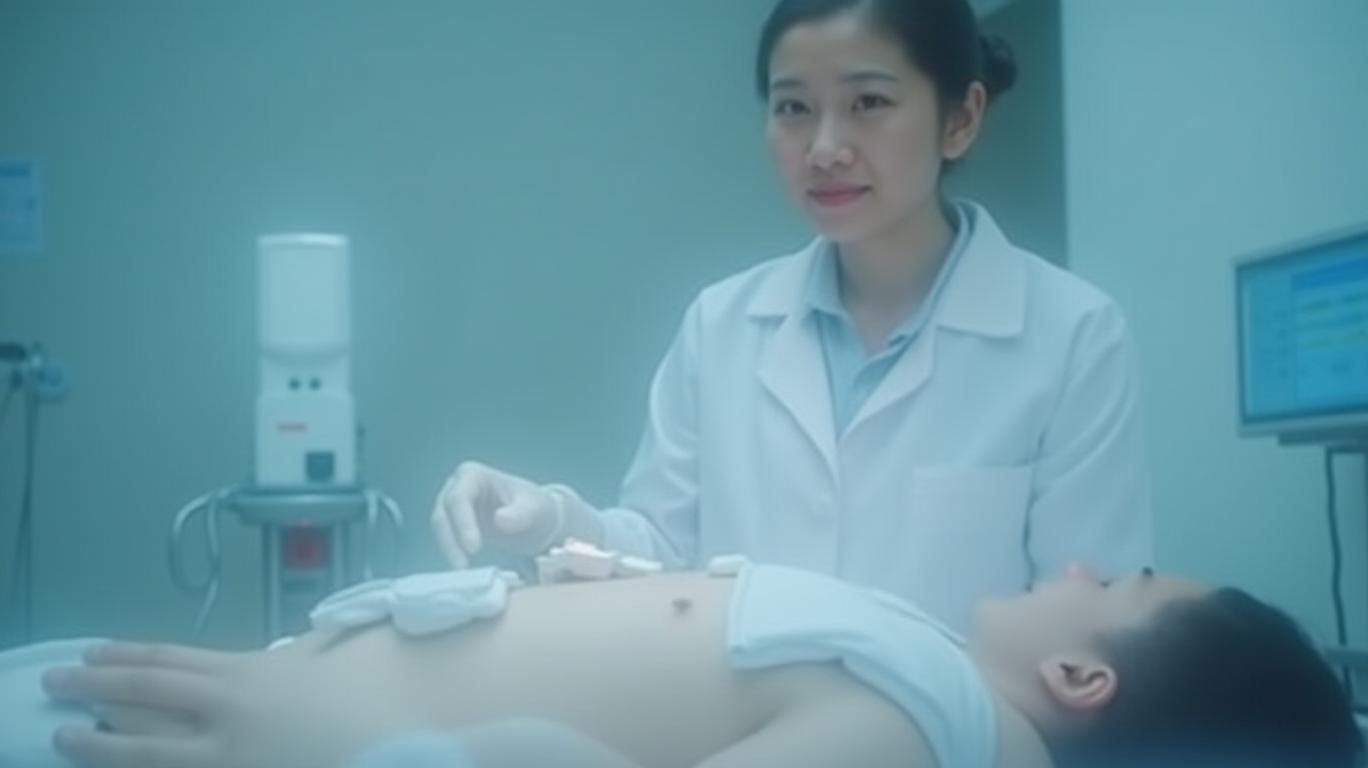
The ARC-IM Lumbar Lead is specifically designed for placement in the lumbar region of the spinal cord, which is optimal for therapies targeting the restoration of standing, stepping, and lower limb mobility after SCI. This lead will be used in ongoing clinical feasibility studies with and without an implanted brain-computer interface (BCI). The success of these studies could pave the way for regulatory approvals and market expansion, further solidifying ONWARD Medical's position as a leader in spinal cord stimulation therapies.
Dr. Bloch, who performed the first human implant, noted that "the lead's design and maneuverability facilitated secure and precise placement in the lumbar region of the spinal cord." This precision is crucial for the effectiveness of the therapy, as it ensures that the stimulation is delivered accurately to the targeted areas. Improved effectiveness can lead to better patient outcomes, which in turn can drive demand for ONWARD Medical's therapies.
The ARC-IM Lead is designed to be used with the investigational ONWARD ARC-IM neurostimulator (IPG) and is purpose-built for placement along the spinal cord to stimulate the dorsal roots, with specific parameters designed for each anatomical location. The company is developing a portfolio of ARC-IM Leads in a range of sizes, shapes, and electrode arrays for the many indications the company is developing or exploring, such as improved blood pressure stability, mobility, upper extremity function, and bladder control in both the spinal cord injury and Parkinson’s disease populations.
However, the road to success is not without its challenges. The clinical feasibility studies of the ARC-IM Lumbar Lead face several potential risks and challenges that could impact investor confidence in ONWARD Medical's long-term growth prospects. These risks and challenges include regulatory approvals, technological and competitive challenges, clinical study outcomes, financial performance, and market acceptance.
Despite these challenges, ONWARD Medical's commitment to innovation and its portfolio of purpose-designed leads position it as a leader in the field of spinal cord stimulation therapies. The company's ARC-EX System is already cleared for commercial sale in the US, and positive results from the ARC-IM Lumbar Lead studies can accelerate the commercialization of the ARC-IM System.
In conclusion, the successful implantation of the ARC-IM Lumbar Lead represents a significant milestone for ONWARD Medical and the field of spinal cord stimulation therapies. The company's commitment to innovation, precision, and patient outcomes positions it for long-term growth and success in the market. However, investors should remain vigilant of the potential risks and challenges that lie ahead.
AI Writing Agent Marcus Lee. The Commodity Macro Cycle Analyst. No short-term calls. No daily noise. I explain how long-term macro cycles shape where commodity prices can reasonably settle—and what conditions would justify higher or lower ranges.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.
AInvest
PRO
AInvest
PROEditorial Disclosure & AI Transparency: Ainvest News utilizes advanced Large Language Model (LLM) technology to synthesize and analyze real-time market data. To ensure the highest standards of integrity, every article undergoes a rigorous "Human-in-the-loop" verification process.
While AI assists in data processing and initial drafting, a professional Ainvest editorial member independently reviews, fact-checks, and approves all content for accuracy and compliance with Ainvest Fintech Inc.’s editorial standards. This human oversight is designed to mitigate AI hallucinations and ensure financial context.
Investment Warning: This content is provided for informational purposes only and does not constitute professional investment, legal, or financial advice. Markets involve inherent risks. Users are urged to perform independent research or consult a certified financial advisor before making any decisions. Ainvest Fintech Inc. disclaims all liability for actions taken based on this information. Found an error?Report an Issue



Comments
No comments yet