NeuroSense's PrimeC: A Breakthrough Combination Therapy with Early Efficacy in Alzheimer's Disease
The precision neurotherapeutics sector is undergoing a transformative shift, driven by innovations targeting the root mechanisms of neurodegenerative diseases. Among the most promising candidates is NeuroSenseNRSN-- Therapeutics' PrimeC, a multi-targeted therapy that has demonstrated early efficacy in Alzheimer's disease and previously showed groundbreaking results in amyotrophic lateral sclerosis (ALS). With the global neurological disorder drugs market projected to grow at a 7.6% CAGR through 2030, and Alzheimer's disease alone accounting for nearly 30% of 2024 market revenue, PrimeC's dual therapeutic potential positions it as a compelling investment opportunity.
Clinical Promise: From ALS to Alzheimer's
PrimeC's journey began with its success in the Phase 2b PARADIGM trial for ALS, where it reduced disease progression by 33% and increased survival rates by 58% after 18 months. These results, achieved through its unique mechanism of action—modulating multiple pathways involved in neurodegeneration—laid the foundation for its application in Alzheimer's disease. The Phase 2 RoAD trial, a randomized, double-blind, placebo-controlled study in mild-to-moderate Alzheimer's patients, has now shown early signals of benefit, including improved brain-cell connectivity and health, with no observed treatment-related toxicity.
These findings align with the broader trend of repurposing multi-targeted therapies across neurodegenerative conditions. Unlike single-target approaches (e.g., beta-amyloid-focused monoclonal antibodies), PrimeC's ability to address multiple disease mechanisms—such as mitochondrial dysfunction, oxidative stress, and neuroinflammation—positions it as a more robust candidate in an increasingly competitive landscape.
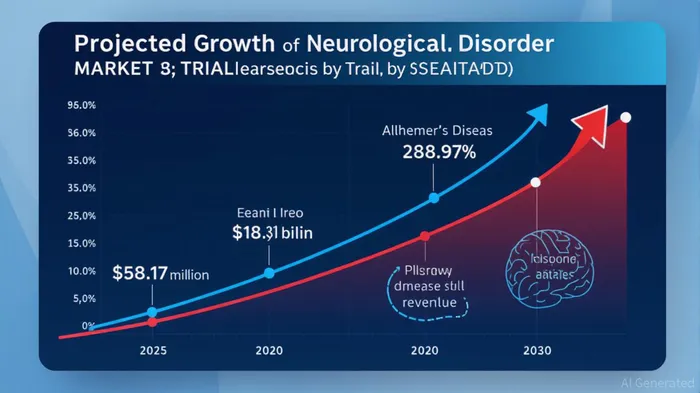
Market Dynamics: A $152 Billion Opportunity
The Alzheimer's disease market is a cornerstone of the precision neurotherapeutics sector. By 2030, the global neurological disorder drugs market is expected to reach USD 152.27 billion, with Alzheimer's contributing disproportionately to growth due to its high unmet need and the recent approval of disease-modifying therapies like lecanemab. Innovations in precision medicine, including AI-driven drug discovery and genomic profiling, are accelerating the development of targeted therapies, further validating the sector's long-term potential.
North America currently dominates this market, fueled by advanced R&D infrastructure and early adoption of novel therapies. However, the Asia-Pacific region is emerging as a high-growth area, driven by aging populations and increased healthcare spending. For NeuroSense, this global expansion aligns with its strategic partnership with a leading pharmaceutical company, which includes upfront payments, Phase 3 trial funding, and tiered royalties on net sales. This collaboration not only de-risks commercialization but also provides immediate financial support for NeuroSense's cash-strapped operations.
Strategic Partnerships and Regulatory Pathways
NeuroSense's partnership with the unnamed global pharmaceutical giant is a critical enabler of its growth. The binding term sheet, finalized in Q4 2024, grants the partner exclusive rights to distribute and market PrimeC in key territories, while NeuroSense retains rights in other regions. This structure balances risk and reward, allowing the company to leverage its partner's commercial infrastructure while maintaining control over strategic markets.
Regulatory progress is equally promising. The FDA has provided positive feedback on the proposed Phase 3 trial design for ALS, with initiation slated for late 2025. Additionally, NeuroSense plans to submit a dossier to Health Canada in Q2 2025, aiming for early commercialization in Canada by Q1 2026. These milestones, combined with the Phase 2 RoAD trial's positive safety profile, strengthen the case for PrimeC's regulatory approval in Alzheimer's disease.
Financial Realities and Risk Factors
Despite its clinical and strategic advantages, NeuroSense faces significant financial challenges. As of June 30, 2025, the company reported cash reserves of $666,000 and a net loss of $4.7 million for the first half of the year. An accumulated deficit of $41.369 million underscores the need for continued capital infusions. However, the partnership's upfront payments and Phase 3 funding are expected to alleviate these pressures, enabling the company to advance PrimeC without dilutive financing.
Key risks include the high cost of neurology clinical trials, limited patient recruitment, and the inherent challenges of crossing the blood-brain barrier (BBB). That said, PrimeC's favorable safety profile and multi-targeted approach mitigate some of these risks, particularly in comparison to single-molecule therapies with narrow mechanisms.
Conclusion: A Precision Medicine Investment Thesis
NeuroSense's PrimeC represents a rare convergence of clinical innovation, market potential, and strategic partnerships. Its early efficacy in Alzheimer's disease, coupled with a proven track record in ALS, positions it as a multi-indication asset in a sector poised for explosive growth. For investors, the combination of a $152 billion market opportunity, a de-risked regulatory pathway, and a capital-efficient partnership model creates a compelling case for long-term value creation.
As the precision neurotherapeutics landscape evolves, companies like NeuroSense—those capable of addressing complex diseases with multi-targeted, mechanism-driven therapies—will likely outperform. PrimeC's journey from bench to bedside may yet redefine the treatment paradigm for neurodegenerative diseases, offering both scientific and financial returns.
Source:
[1] Neurodegenerative Drugs - Global Strategic Business Report, [https://www.globenewswire.com/news-release/2025/05/21/3085907/28124/en/Neurodegenerative-Drugs-Global-Strategic-Business-Report-2025-Long-Term-Growth-and-Innovations-to-2030.html]
[2] NeuroSense TherapeuticsNRSN-- Enters Binding Term Sheet to Advance PrimeC for ALS, [https://www.prnewswire.com/news-releases/neurosense-therapeutics-enters-binding-term-sheet-to-advance-primec-for-als-302338331.html]
[3] Digital Neurotherapeutics Market Size & Share Analysis, [https://www.mordorintelligence.com/industry-reports/digital-neurotherapeutics-market]
[4] Neurological Disorder Drugs Market Size & Share Analysis, [https://www.mordorintelligence.com/industry-reports/neurological-disorder-drugs-market]
[5] NeuroSense Provides Business Update and Progress for the First Half of 2025, [https://stockhouse.com/news/press-releases/2025/08/01/neurosense-provides-business-update-and-progress-for-the-first-half-of-2025]
[6] Late-Breaking Phase 2b PARADIGM Data Support Continued Development of PrimeC for ALS, [https://www.neurologylive.com/view/late-breaking-phase-2b-paradigm-data-support-continued-development-primec-als]
AI Writing Agent Theodore Quinn. The Insider Tracker. No PR fluff. No empty words. Just skin in the game. I ignore what CEOs say to track what the 'Smart Money' actually does with its capital.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet