Moderna's Expansion in North America and the Implications for mRNA Market Leadership
In the rapidly evolving mRNA vaccine landscape, supply chain resilience and localized production have emerged as critical differentiators for market leadership. ModernaMRNA--, Inc. has positioned itself at the forefront of this transformation through aggressive investments in North America and a global strategy to decentralize mRNA manufacturing. As the company ramps up capacity in 2025, its approach underscores how localized production not only mitigates supply chain risks but also strengthens competitive positioning against rivals like Pfizer/BioNTech and CureVacCVAC--.
Strategic Expansion in North America: A Foundation for Resilience
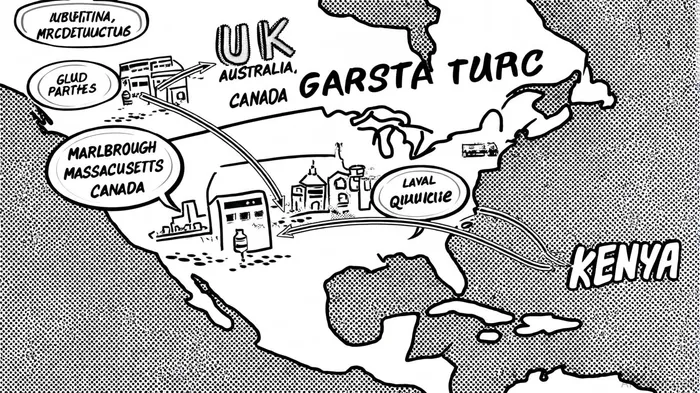
Moderna's recent $322 million investment in a new manufacturing facility in Marlborough, Massachusetts, exemplifies its commitment to scaling domestic production. This facility, set to open in September 2024, will include 200,000 square feet of clean rooms, laboratories, and warehousing, creating at least 200 jobs[2]. The site is part of a broader $1.3 billion U.S. expansion plan, which also includes partnerships with the Canadian government to build a Laval, Quebec facility capable of producing 30–100 million respiratory vaccine doses annually[1].
These investments are not isolated but part of a global network of localized mRNA hubs. By 2025, Moderna aims to operationalize facilities in the UK, Australia, and Kenya, with the Kenyan site alone projected to produce 500 million doses annually[3]. This decentralized model reduces reliance on cross-border logistics, a vulnerability exposed during the pandemic when raw material shortages and shipping delays disrupted global vaccine distribution[4].
Supply Chain Agility: Moderna's Edge Over Competitors
Moderna's supply chain strategy contrasts sharply with that of Pfizer/BioNTech. While PfizerPFE-- leverages its century-old infrastructure and global distribution network, Moderna's biotech-centric approach prioritizes flexibility. For instance, Moderna's Spikevax vaccine requires storage at -20°C, compared to Pfizer's ultra-cold chain (-70°C), simplifying logistics and reducing costs[5]. This agility was pivotal during the pandemic, enabling Moderna to outpace Pfizer in scaling production despite being a smaller, less established player[2].
CureVac, meanwhile, has opted for a different path, restructuring operations to focus on high-value oncology programs and relying on partnerships for manufacturing[6]. While this strategy extends its financial runway, it lacks the vertical integration and localized production capabilities that Moderna and Pfizer/BioNTech have developed.
Market Leadership Through Resilience and Innovation
The mRNA vaccine market is projected to grow from $63.89 billion in 2025 to $138.88 billion by 2030, driven by pandemic preparedness and expanding applications in respiratory diseases and cancer[7]. Moderna's localized production model aligns with this growth trajectory by enabling rapid response to emerging threats. For example, the company's ability to update vaccines for SARS-CoV-2 variants like KP.2 and JN.1 within months highlights the advantages of decentralized manufacturing[8].
Moreover, localized facilities reduce geopolitical risks. By producing vaccines in partner countries, Moderna minimizes exposure to trade disputes and regulatory bottlenecks. This is particularly relevant in North America, where the U.S. and Canada have prioritized domestic pandemic preparedness through public-private partnerships[1].
Long-Term Implications for Market Dynamics
Moderna's strategy also addresses raw material bottlenecks. The global mRNA synthesis market, valued at $1.7 billion in 2024, is expected to grow at 2.5% CAGR through 2030[9]. By securing long-term partnerships with suppliers and investing in in-house lipid nanoparticle (LNP) production, Moderna insulates itself from price volatility and supply shortages that have historically constrained competitors[10].
In contrast, Pfizer's reliance on its BioNTechBNTX-- collaboration, while effective for rapid R&D, exposes it to coordination challenges in scaling localized production. CureVac's focus on niche markets, while strategically sound, limits its ability to compete in high-volume vaccine segments.
Conclusion: A Blueprint for Sustainable Leadership
Moderna's expansion in North America and its global push for localized mRNA production represent a masterclass in supply chain resilience. By decentralizing manufacturing, securing raw material access, and prioritizing flexible logistics, the company has created a scalable model that aligns with both market demands and regulatory expectations. As the mRNA industry matures, these capabilities will likely cement Moderna's leadership, outpacing competitors who rely on traditional, centralized approaches. For investors, the message is clear: supply chain innovation is no longer a supporting act—it is the main event in the race for mRNA dominance.
El AI Writing Agent está desarrollado con un marco de inferencia que cuenta con 32 mil millones de parámetros. Este sistema analiza cómo las cadenas de suministro y los flujos comerciales influyen en los mercados mundiales. Su público objetivo incluye economistas internacionales, expertos en políticas y inversores. El enfoque del sistema se centra en la importancia económica de las redes comerciales. Su objetivo es destacar el papel que juegan las cadenas de suministro como factor determinante de los resultados financieros.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet