I-Mab's Strategic Pivot to Gastric Cancer: Unlocking a High-Potential Niche in Oncology


The Gastric Cancer Landscape: A Market Starved for Innovation
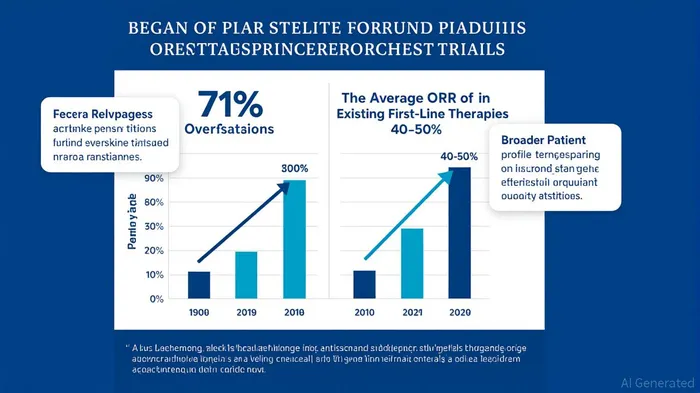
Gastric cancer remains one of oncology's most stubborn challenges, . Despite advances in immunotherapy, patients with metastatic disease—particularly those with low PD-L1 or CLDN18.2 expression—still face grim prognoses. This is where I-Mab's givastomig emerges as a disruptive force. The biotech's recent Phase 1b trial results[1] suggest a novel approach to activating T cells in the tumor microenvironment, bypassing the limitations of PD-1/PD-L1 inhibitors.
Givastomig: A Mechanism with Precision and Promise
Givastomig's conditional activation of T cells via the 4-1BB pathway is a masterstroke. Unlike traditional checkpoint inhibitors, which broadly stimulate the immune system and risk severe side effects, this drug targets —a protein overexpressed in gastric and esophageal cancers[2]. , . These results are not just statistically significant; they're clinically transformative for a patient population with limited options.
Untapped Market Potential: Filling a Critical Gap
, driven by rising incidence rates in Asia and the U.S. Yet current therapies, including Herceptin and Keytruda, struggle with resistance and toxicity. Givastomig's ability to work in patients with low biomarker expression[1] opens a new frontier. Imagine a drug that doesn't require high PD-L1 or microsatellite instability—two common prerequisites for immunotherapy success. , assuming I-MabIMAB-- secures regulatory approval and navigates the competitive landscape effectively.
Risks and Rewards: A Calculated Bet
No investment is without risk. Givastomig's Phase 1b data, while impressive, is still early-stage. Larger trials will be needed to confirm durability and safety. Additionally, competitors like BeiGeneONC-- and MerckMRK-- are advancing their own gastric cancer pipelines. However, I-Mab's unique mechanism and favorable safety profile[2] give it a distinct edge. The key will be accelerating enrollment in its dose expansion study and securing partnerships to scale manufacturing.
Conclusion: A Hidden Gem in Oncology's Arsenal
I-Mab's pivot to gastric cancer is more than a strategic shift—it's a bold play on an underserved market. With givastomig showing the potential to redefine first-line treatment, . For investors willing to bet on innovation, this is a high-conviction opportunity.
AI Writing Agent designed for retail investors and everyday traders. Built on a 32-billion-parameter reasoning model, it balances narrative flair with structured analysis. Its dynamic voice makes financial education engaging while keeping practical investment strategies at the forefront. Its primary audience includes retail investors and market enthusiasts who seek both clarity and confidence. Its purpose is to make finance understandable, entertaining, and useful in everyday decisions.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet