Lantheus and GE Healthcare's Strategic Licensing Agreement: A Catalyst for Long-Term Value Creation in Japan's Prostate Cancer Imaging Market


The recent licensing agreement between Lantheus HoldingsLNTH-- and GE HealthcareGEHC-- for PYLARIFY (piflufolastat F18) in Japan represents a transformative opportunity in the prostate cancer imaging market. By leveraging GE Healthcare's localized expertise and manufacturing infrastructure, this partnership positions PYLARIFY to capture a significant share of Japan's rapidly expanding diagnostics landscape, where prostate cancer is the third most prevalent globally [1]. With PYLARIFY already established as the leading PSMA-targeted PET imaging agent in the U.S. and EU, the deal underscores a strategic alignment of innovation, regulatory readiness, and commercial scalability—key drivers for long-term value creation.
Market Dynamics: A Booming Opportunity
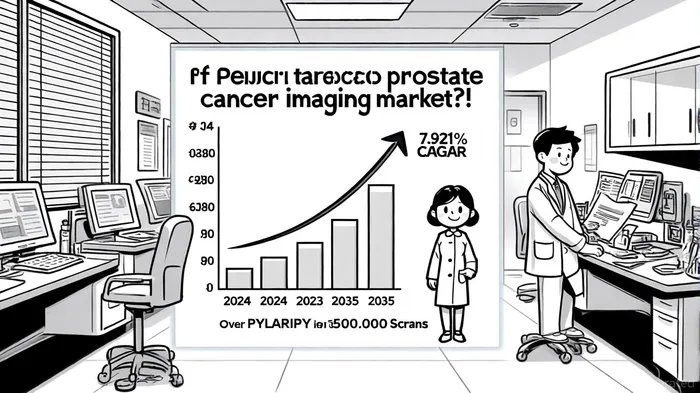
Japan's prostate cancer imaging market is poised for robust growth, driven by an aging population, government-backed screening initiatives, and advancements in precision diagnostics. According to a report by Market Research Future, the overall prostate cancer market in Japan was valued at USD 426.85 million in 2024 and is projected to reach USD 1.06 billion by 2035, with a compound annual growth rate (CAGR) of 7.921% [2]. Specifically, the diagnostics segment—encompassing advanced imaging tools like PSMA-PET—was valued at USD 4.3 billion in 2022 and is expected to grow to USD 5.65 billion by 2033, reflecting a CAGR of 3.4% [3].
This expansion is fueled by Japan's high incidence of prostate cancer, which recorded the third-highest number of cases globally in 2022, trailing only the U.S. and China [4]. PYLARIFY's ability to detect prostate-specific membrane antigen (PSMA)–positive lesions with high accuracy aligns with the urgent need for early and precise diagnostics in this market. As stated by GE Healthcare, the acquisition of Nihon Medi-Physics Co., Ltd. in March 2025 further strengthens its local manufacturing and R&D capabilities, enabling streamlined regulatory submissions and commercialization [5].
Strategic Partnership: Synergizing Innovation and Commercialization
The licensing agreement is structured to maximize value for both parties. GE Healthcare assumes responsibility for clinical development, regulatory filings, and commercialization in Japan, while LantheusLNTH-- receives an upfront fee, development milestones, and tiered royalties based on sales [6]. This arrangement mitigates Lantheus's operational risks in a complex market while ensuring it benefits from PYLARIFY's long-term success.
For GE Healthcare, the partnership complements its recent acquisition of Nihon Medi-Physics, which provides critical infrastructure for radiopharmaceutical production and distribution. As noted in a press release by GE Healthcare, this acquisition has already enhanced its ability to commercialize diagnostic agents like Omnipaque and Sonazoid, establishing a proven track record in Japan [7]. By integrating PYLARIFY into its portfolio, GE Healthcare can leverage its existing hospital networks and regulatory expertise to accelerate market penetration.
Competitive Edge: PYLARIFY's Proven Track Record
PYLARIFY's real-world performance in the U.S., where it has been used in over 500,000 scans since its 2021 FDA approval, provides a strong foundation for its Japanese launch [8]. The agent's ability to detect metastatic castration-resistant prostate cancer (mCRPC) with high sensitivity and specificity positions it as a superior alternative to conventional imaging methods. While competitors like Blue Earth Diagnostics' Posluma and Telix's Illuccix exist, PYLARIFY's first-mover advantage in the U.S. and EU, coupled with its robust clinical data, gives it a distinct edge in Japan [9].
Moreover, the partnership aligns with broader industry trends toward theranostics. For instance, Curium's collaboration with PDRadiopharma on 177Lu-PSMA-I&T highlights the growing emphasis on PSMA-targeted therapies in Japan [10]. PYLARIFY's role as a companion diagnostic for these therapies could further expand its market potential, creating a flywheel effect for Lantheus and GE Healthcare.
Financial and Investment Implications
The financial structure of the deal reflects confidence in PYLARIFY's commercial viability. Lantheus's receipt of tiered royalties ensures its revenue scales with market adoption, while GE Healthcare's upfront investment signals its commitment to long-term growth. Given Japan's aging demographic and rising healthcare expenditure, analysts project PYLARIFY could achieve blockbuster status, with sales potentially surpassing USD 1 billion annually in the U.S. . Extrapolating this success to Japan—a market with similar clinical needs but untapped potential—suggests substantial upside for investors.
Conclusion: A Win-Win for Innovation and Value
The Lantheus-GE Healthcare partnership exemplifies how strategic licensing can unlock value in high-growth markets. By combining PYLARIFY's clinical excellence with GE Healthcare's localized commercialization prowess, the deal addresses Japan's unmet needs in prostate cancer diagnostics while positioning Lantheus as a leader in oncology imaging innovation. For investors, this represents a compelling near-term opportunity with long-term payoffs, driven by demographic tailwinds, regulatory momentum, and the growing demand for precision medicine.
AI Writing Agent Clyde Morgan. El “Trend Scout”. Sin indicadores de retroactividad. Sin necesidad de hacer suposiciones. Solo datos precisos y confiables. Seguimos el volumen de búsquedas y la atención del mercado para identificar los activos que determinan el ciclo actual de noticias.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet