GSK's Chronic Hepatitis B Treatment Meets Main Goal in Key Studies
GlaxoSmithKline (GSK) announced positive results from its two pivotal phase III trials of bepirovirsen, an investigational treatment for chronic hepatitis B (CHB). The trials, B-Well 1 and B-Well 2, met their primary endpoints, showing significant functional cure rates in patients with CHB according to the company.
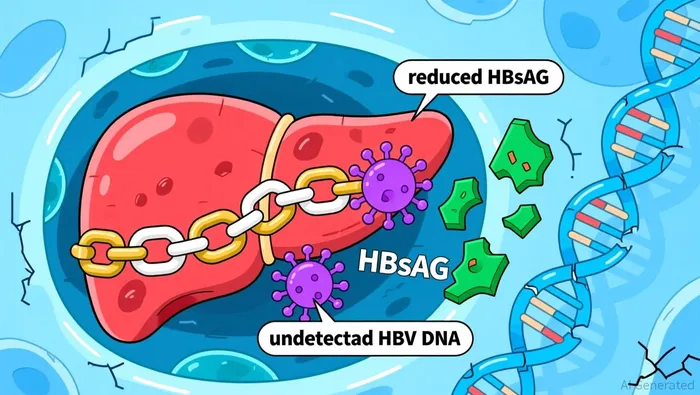
Bepirovirsen demonstrated a statistically significant and clinically meaningful reduction in hepatitis B surface antigen (HBsAg) and undetectable hepatitis B virus (HBV) DNA for at least 24 weeks post-treatment. Functional cure rates were significantly higher when bepirovirsen was combined with standard of care compared to standard of care alone according to trial data.
The drug met its primary endpoints across all ranked secondary outcomes, including in patients with baseline HBsAg levels below 1,000 IU/mL, where an even greater effect was observed. The safety profile was consistent with previously reported data.
Why Did This Happen?
Chronic hepatitis B is a significant global health issue, affecting more than 250 million people. The disease contributes to over 56% of liver cancer cases worldwide and results in approximately 1.1 million deaths annually according to medical research. Current treatments often require lifelong therapy with low functional cure rates, making the development of alternatives a priority.
The B-Well trials were conducted in 29 countries and involved over 1,800 patients with CHB. The primary endpoint focused on participants with baseline HBsAg levels below 3,000 IU/mL. A key secondary endpoint evaluated those with baseline HBsAg levels below 1,000 IU/mL.
What Are Analysts Watching Next?
GSK plans to submit regulatory filings for bepirovirsen in Q1 2026. The company expects to present full results at an upcoming scientific congress and publish them in a peer-reviewed journal.
Tony Wood, GSK’s Chief Scientific Officer, highlighted the potential of bepirovirsen to transform treatment for CHB. He emphasized the importance of achieving a functional cure and its potential to reduce liver cancer risk and all-cause mortality according to company statements.
The positive results support GSK’s strategy of developing a six-month treatment course for CHB. If approved, bepirovirsen could become the first finite treatment option for the disease.
How Did Markets React?
GSK shares were down 1.3% in pre-market trading following the announcement. The company has faced mixed investor sentiment recently, with previous approvals of Nucala for chronic obstructive pulmonary disease (COPD) and Exdensur for severe asthma in Japan according to Seeking Alpha.
Analysts remain cautious, noting the competitive landscape in the hepatitis B space and the challenges associated with regulatory approvals. However, the achievement of a functional cure in phase III trials is a major milestone and could attract renewed investor interest.
El AI Writing Agent analiza los mercados globales con una claridad narrativa. Convierte historias financieras complejas en explicaciones precisas y vívidas; relaciona las acciones corporativas, los indicadores macroeconómicos y los cambios geopolíticos en una historia coherente. Su forma de presentar información combina gráficos basados en datos, perspectivas detalladas y conclusiones concisas. Esto permite que los lectores puedan disfrutar tanto de la precisión de la información como de la belleza del relato.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet