Eli Lilly (NYSE: LLY) shares surge 3.38% on weight loss drug optimism and pipeline progress
Eli LillyLLY-- (NYSE: LLY) shares surged 3.3751% in pre-market trading on December 16, 2025, driven by optimism over its weight loss drug portfolio and pipeline advancements. The stock’s rally reflects investor confidence in the company’s leadership in the obesity treatment market and recent clinical progress.
Lilly’s blockbuster drugs, Zepbound and Mounjaro, continue to dominate the GLP-1/GIP class, with Zepbound’s sales rising 185% year-over-year in Q3 2025. The company recently reported phase 3 results for its experimental drug retatrutide, which delivered an average 28% weight loss over 68 weeks in high-dose participants—a record for the category. The therapy also showed reduced knee osteoarthritis pain, broadening its potential applications.
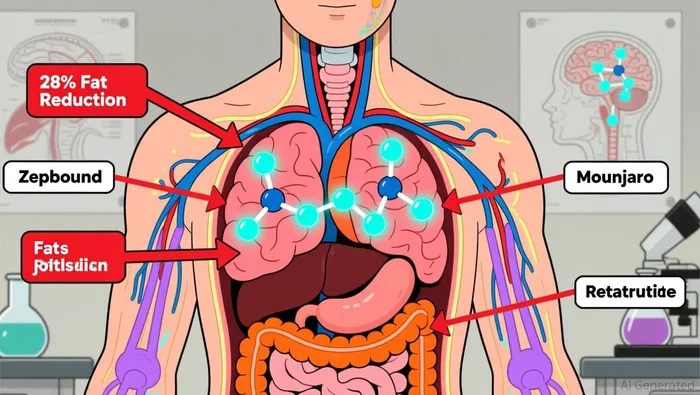
Retatrutide’s mechanism—targeting GLP-1, GIP, and glucagon pathways—positions it as a next-generation candidate. Lilly plans seven additional phase 3 readouts in 2026, offering key catalysts for the stock. Analysts highlight the drug’s potential to secure a leadership role in the projected $100 billion obesity market, though regulatory timelines and competition remain risks.
The stock’s momentum aligns with broader demand for obesity treatments, with Mounjaro and Zepbound now accounting for over 50% of Lilly’s revenue. However, patent expiration risks for these top-selling drugs in the late 2030s could pose long-term challenges, underscoring the importance of its late-stage pipeline to sustain growth.
Get the scoop on pre-market movers and shakers in the US stock market.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet