Eisai-Biogen's Injectable Alzheimer's Drug Gets FDA Approval
ByAinvest
Friday, Aug 29, 2025 5:04 pm ET1min read
BIIB--
Leqembi, developed by Eisai and Biogen, is an injectable monoclonal antibody that targets amyloid beta (Aβ) and tau proteins, which are key factors in the development of Alzheimer's disease. The drug has been shown to slow the progression of the disease in clinical trials, offering hope to patients and their families.
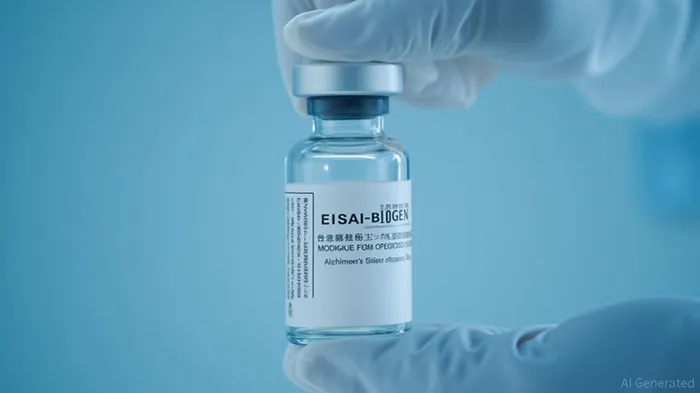
The FDA's approval of the injectable version of Leqembi marks a significant advancement in the treatment of Alzheimer's disease. The injectable formulation, known as LEQEMBI IQLIK, is a subcutaneous autoinjector that delivers a 360 mg weekly injection in approximately 15 seconds. This at-home alternative offers patients a more convenient and less intrusive way to administer the drug, which can be particularly beneficial for those who have been undergoing intravenous infusions for 18 months.
Clinical trials have demonstrated that the 360 mg weekly subcutaneous dose maintains comparable clinical and biomarker benefits to continued intravenous dosing. The safety profile of the subcutaneous injection is also notable, with less than 1% systemic reactions compared to approximately 26% with intravenous infusions.
Biogen and Eisai have committed to ensuring that appropriate patients have access to Leqembi. The companies have announced several support programs, including dedicated patient navigators to help with treatment and coverage, and a patient assistance program to provide the drug at no cost for eligible uninsured and underinsured patients.
The FDA approval of LEQEMBI IQLIK is expected to increase treatment accessibility and adherence for Alzheimer's patients. It will also reduce healthcare resource utilization by freeing up infusion capacity for new patients while enabling continued treatment for maintenance patients without the burden of regular infusion center visits.
Biogen, a leading therapeutic products company, has seen significant revenue growth in recent years, with a substantial portion of its net sales coming from the sale of medicines, including treatments for multiple sclerosis, chronic psoriasis, and cancers. The company also generates revenue through royalties and partnership agreements. The approval of LEQEMBI IQLIK is expected to further bolster Biogen's financial performance and position in the Alzheimer's treatment market.
References:
[1] https://www.reuters.com/business/healthcare-pharmaceuticals/fda-recommends-more-monitoring-alzheimers-patients-eisai-biogens-drug-leqembi-2025-08-28/
[2] https://www.stocktitan.net/news/BIIB/fda-approves-leqembi-iqliktm-lecanemab-irmb-subcutaneous-injection-r9k723nqvbc4.html
The US FDA has approved an injectable version of Eisai-Biogen's Alzheimer's drug. Biogen is a leading therapeutic products company with a significant portion of its net sales coming from the sale of medicines, including treatments for multiple sclerosis, chronic psoriasis, and cancers. The company also generates revenue through royalties and partnership agreements.
The U.S. Food and Drug Administration (FDA) has approved an injectable version of Eisai and Biogen's Alzheimer's drug, Leqembi. This approval follows a significant milestone in the ongoing efforts to combat Alzheimer's disease, a progressive and devastating neurodegenerative condition.Leqembi, developed by Eisai and Biogen, is an injectable monoclonal antibody that targets amyloid beta (Aβ) and tau proteins, which are key factors in the development of Alzheimer's disease. The drug has been shown to slow the progression of the disease in clinical trials, offering hope to patients and their families.
The FDA's approval of the injectable version of Leqembi marks a significant advancement in the treatment of Alzheimer's disease. The injectable formulation, known as LEQEMBI IQLIK, is a subcutaneous autoinjector that delivers a 360 mg weekly injection in approximately 15 seconds. This at-home alternative offers patients a more convenient and less intrusive way to administer the drug, which can be particularly beneficial for those who have been undergoing intravenous infusions for 18 months.
Clinical trials have demonstrated that the 360 mg weekly subcutaneous dose maintains comparable clinical and biomarker benefits to continued intravenous dosing. The safety profile of the subcutaneous injection is also notable, with less than 1% systemic reactions compared to approximately 26% with intravenous infusions.
Biogen and Eisai have committed to ensuring that appropriate patients have access to Leqembi. The companies have announced several support programs, including dedicated patient navigators to help with treatment and coverage, and a patient assistance program to provide the drug at no cost for eligible uninsured and underinsured patients.
The FDA approval of LEQEMBI IQLIK is expected to increase treatment accessibility and adherence for Alzheimer's patients. It will also reduce healthcare resource utilization by freeing up infusion capacity for new patients while enabling continued treatment for maintenance patients without the burden of regular infusion center visits.
Biogen, a leading therapeutic products company, has seen significant revenue growth in recent years, with a substantial portion of its net sales coming from the sale of medicines, including treatments for multiple sclerosis, chronic psoriasis, and cancers. The company also generates revenue through royalties and partnership agreements. The approval of LEQEMBI IQLIK is expected to further bolster Biogen's financial performance and position in the Alzheimer's treatment market.
References:
[1] https://www.reuters.com/business/healthcare-pharmaceuticals/fda-recommends-more-monitoring-alzheimers-patients-eisai-biogens-drug-leqembi-2025-08-28/
[2] https://www.stocktitan.net/news/BIIB/fda-approves-leqembi-iqliktm-lecanemab-irmb-subcutaneous-injection-r9k723nqvbc4.html
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.
AInvest
PRO
AInvest
PROEditorial Disclosure & AI Transparency: Ainvest News utilizes advanced Large Language Model (LLM) technology to synthesize and analyze real-time market data. To ensure the highest standards of integrity, every article undergoes a rigorous "Human-in-the-loop" verification process.
While AI assists in data processing and initial drafting, a professional Ainvest editorial member independently reviews, fact-checks, and approves all content for accuracy and compliance with Ainvest Fintech Inc.’s editorial standards. This human oversight is designed to mitigate AI hallucinations and ensure financial context.
Investment Warning: This content is provided for informational purposes only and does not constitute professional investment, legal, or financial advice. Markets involve inherent risks. Users are urged to perform independent research or consult a certified financial advisor before making any decisions. Ainvest Fintech Inc. disclaims all liability for actions taken based on this information. Found an error?Report an Issue

Comments
No comments yet