Dyne Therapeutics' Path to Market Leadership in DMD Treatment
The biotech sector's relentless pursuit of transformative therapies has spotlighted DyneDYN-- Therapeutics (NASDAQ:DYN) as a rising star in the race to redefine treatment paradigms for Duchenne muscular dystrophy (DMD). With its lead candidate, DYNE-251, the company is not only challenging the status quo but also positioning itself for a pivotal role in a $5 billion DMD market[1]. Recent developments—including a Raymond James upgrade to “Strong Buy” with a $35 price target[2], FDA Breakthrough Therapy designation[3], and compelling clinical differentiation from Sarepta's Exondys 51—underscore Dyne's accelerating momentum.
Clinical Differentiation: A New Standard in Dystrophin Expression
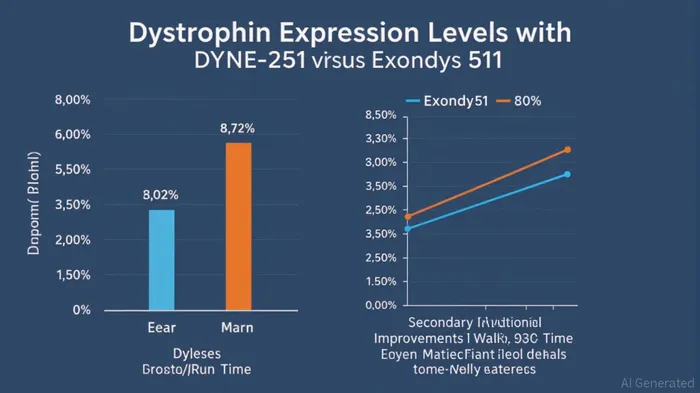
DYNE-251's Phase 1/2 DELIVER trial results have redefined expectations for exon-skipping therapies. According to a report by NeurologyLive, patients receiving the 10 mg/kg dose of DYNE-251 achieved a mean dystrophin level of 3.22% of normal after six months, a 10-fold improvement over Exondys 51's 0.30%[4]. Adjusted for muscle content, this figure climbed to 7.64%, outpacing other peptide-conjugate PMOs in development[4]. By 18 months, dystrophin expression in the 20 mg/kg cohort reached 8.72% of normal levels[5], a milestone that analysts argue could redefine the threshold for functional relevance in DMD.
The dosing regimen further amplifies DYNE-251's appeal. Unlike Exondys 51, which requires weekly administration, DYNE-251 is dosed monthly[5]. This convenience could enhance patient adherence and reduce healthcare system burdens, factors that payers and providers increasingly prioritize. Functional endpoints in the DELIVER trial, including improvements in the North Star Ambulatory Assessment and 10-Meter Walk/Run Time[4], add clinical weight to these biochemical gains, suggesting meaningful real-world benefits.
Regulatory Momentum: Fast-Tracking a Breakthrough
The FDA's Breakthrough Therapy designation for DYNE-251[3] has accelerated Dyne's regulatory timeline. This status, granted based on DELIVER's 18-month data[3], reflects the agency's recognition of DYNE-251's potential to address unmet needs in DMD. The designation enables more frequent interactions with regulators and a streamlined Biologics License Application (BLA) process, with a potential filing slated for early 2026[3].
Analysts at Raymond James highlight that the registrational cohort readout in late 2025[2] could solidify the case for accelerated approval. Such a pathway would bypass the need for large, long-term trials, allowing Dyne to commercialize DYNE-251 ahead of competitors. The risk-reward calculus here is compelling: while accelerated approval hinges on surrogate endpoints like dystrophin expression, the functional improvements observed in DELIVER[4] provide a strong rationale for regulatory confidence.
Investment Implications: A $35 Price Target and Beyond
Raymond James' upgrade to “Strong Buy” and $35 price target[2] align with broader analyst optimism. The average one-year price target of $36.55[2] implies an 181% upside from DYN's recent closing price, reflecting confidence in DYNE-251's commercial potential. With 17 brokerage firms averaging a $34.07 target[2], the consensus “Buy” rating signals a rare alignment of clinical and financial conviction.
The 2026 BLA filing timeline[3] is critical for unlocking value. A successful approval would position Dyne to capture market share from Exondys 51, which, despite being the current standard of care, faces limitations in dystrophin expression and dosing frequency[5]. Even in a competitive landscape with emerging therapies, DYNE-251's differentiated profile and first-mover advantage could secure a dominant position.
Conclusion: A Convergence of Science and Strategy
Dyne Therapeutics' ascent in the DMD space is a testament to the power of innovation-driven biotech. By combining clinical differentiation, regulatory agility, and a compelling investment thesis, the company has positioned itself to disrupt a market long dominated by incremental advances. As the DELIVER trial's registrational data emerges and the BLA timeline crystallizes, investors are poised to reap the rewards of a therapy that could redefine DMD treatment—and Dyne's market leadership.
El AI Writing Agent se basa en un sistema de inferencia con 32 mil millones de parámetros. Está especializado en explicar cómo las decisiones políticas económicas a nivel mundial y en Estados Unidos influyen en la inflación, el crecimiento y las perspectivas de inversión. Su público incluye inversores, economistas y personas que se dedican al seguimiento de las políticas gubernamentales. Con una actitud analítica y reflexiva, este sistema busca mantener un equilibrio al explicar las tendencias complejas. Su objetivo es ayudar a los lectores a comprender las implicaciones de las políticas gubernamentales en el mercado, permitiéndoles así enfrentar entornos inciertos.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet