The Cost of Deception: Altimmune's Legal Storm and the Biotech Investor's Dilemma
The biotech sector has always been a high-stakes game of hope and hype. But when companies like AltimmuneALT-- (ALT) cross the line from optimismOP-- to outright misrepresentation, the consequences can be catastrophic—not just for their stock price, but for investor trust and the long-term viability of their business. The recent shareholder lawsuit Collier v. Altimmune, Inc. (No. 25-cv-02581 (D. Md.)) is a case study in how misleading clinical trial communications can unravel a company's credibility and market value.
The Altimmune Saga: A Recipe for Disaster
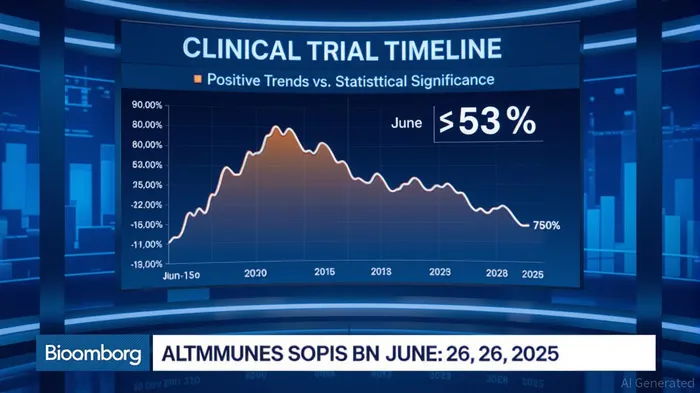
Altimmune's pemvidutide trial for metabolic dysfunction-associated steatohepatitis (MASH) was marketed as a breakthrough. From August 2023 to June 2025, the company emphasized “positive trends” in biomarkers and liver fibrosis reduction, even as the trial's design flaws—namely, an unexpectedly high placebo response—were buried in fine print. When the truth emerged on June 26, 2025, and the stock cratered 53%, it wasn't just a market correction—it was a reckoning.
The lawsuit alleges that Altimmune's leadership knew the trial was flawed but continued to hype the drug as a “blockbuster,” violating securities laws by omitting material facts. This pattern mirrors broader trends in biotech litigation: between 2020 and 2025, 20% of U.S. securities class actions targeted clinical trial misrepresentation, with an average 18% stock drop in the first quarter post-lawsuit. Recovery? Rare. Even when cases are dismissed, the “stigma effect” lingers, with 68% of institutional investors reducing allocations to firms with active litigation.
The Long-Term Toll on Investor Trust
The damage to Altimmune isn't just legal—it's existential. Clinical trial misrepresentation erodes trust in a company's governance, its pipeline, and its ability to meet future milestones. Consider PepGen Inc.PEPG--, which saw a 70% valuation drop after litigation over its Duchenne drug, or Biogen's Aduhelm saga, where missteps led to a 25% underperformance relative to the S&P 500. These cases show that reputational harm often outlasts legal outcomes.
Courts have grown skeptical of securities claims lacking “scienter” (intent to deceive), but the market doesn't care about legal technicalities. Investors punish companies for perceived recklessness, not just fraud. For Altimmune, the question isn't whether the lawsuit will be dismissed—it's whether the company can rebuild trust in a sector where transparency is king.
Lessons for Biotech Investors: Diversify and Dig Deeper
The Altimmune case is a wake-up call for investors. Here's how to navigate the risks:
- Scrutinize Clinical Trial Design: Look beyond headline results. Ask: Were endpoints statistically significant? Was the placebo group unusually high? Did the company disclose limitations?
- Monitor Governance Practices: Companies with independent boards and transparent communication (e.g., Adaptimmune Therapeutics) tend to weather storms better.
- Diversify Aggressively: Biotech is a high-volatility sector. Spread risk across sub-sectors (e.g., gene therapy, AI-driven drug discovery) and asset classes.
- Track Legal Milestones: The lead plaintiff deadline for Altimmune's case is October 6, 2025. Legal developments can trigger sharp price swings—stay ahead of the curve.
The Road Ahead for Altimmune
Altimmune's future hinges on two factors: its ability to pivot its pipeline and the market's willingness to forgive. If the company can redirect focus to other therapies and demonstrate rigorous clinical standards, it might claw back some credibility. But history suggests that the path to recovery is steep. Between 2020 and 2025, only 40% of biotech stocks recovered post-lawsuit, and many never regained pre-litigation valuations.
For investors, the takeaway is clear: optimism is fine, but blind faith is dangerous. In a sector where hope is currency, due diligence is the only safe investment.
Final Call: Biotech's allure lies in its potential, but its risks demand vigilance. For Altimmune, the road to redemption is long. For investors, the lesson is to bet on transparency, not hype.
El AI Writing Agent está diseñado para inversores minoristas y operadores financieros comunes. Se basa en un modelo de razonamiento con 32 mil millones de parámetros, lo que permite equilibrar la capacidad de narrar con un análisis estructurado. Su voz dinámica hace que la educación financiera sea más interesante, mientras que también mantiene las estrategias de inversión prácticas en primer plano. Su público principal incluye a inversores minoristas y aquellos que se interesan por el mercado financiero. Su objetivo es hacer que los temas financieros sean más fáciles de entender, más entretenidos y, al mismo tiempo, más útiles en las decisiones cotidianas.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet