Arvinas' PROTAC-Driven Oncology Play: Assessing the Investment Potential of Next-Gen Immuno-Oncology Synergy
The biotech sector's fascination with protein degradation technologies has intensified in 2025, with ArvinasARVN-- (NASDAQ: ARVN) emerging as a pivotal player in the race to redefine cancer treatment through PROTAC-enabled combination therapies. As the targeted protein degradation (TPD) market expands-projected to grow at a 20.75% CAGR to $1.6 billion by 2030-Arvinas' strategic focus on synergistic oncology regimens positions it at the intersection of innovation and commercial viability. This analysis evaluates the company's emerging combination therapy strategy, partnerships, and market dynamics to gauge its investment potential in the next-generation immuno-oncology landscape.
PROTAC Synergy in Action: ARV-393 and the Lymphoma Opportunity
Arvinas' BCL6 degrader, ARV-393, has become a cornerstone of its oncology pipeline, with preclinical data underscoring its potential to transform treatment paradigms for aggressive lymphomas. According to a report by the company, ARV-393 demonstrated complete tumor regressions in preclinical models of diffuse large B-cell lymphoma (DLBCL) when combined with glofitamab, a CD20×CD3 bispecific antibody. These results, presented at the 2025 American Society of Hematology (ASH) Annual Meeting, revealed synergistic antitumor activity, with tumor growth inhibition (TGI) exceeding 80% in combination cohorts compared to 36–38% for monotherapies.
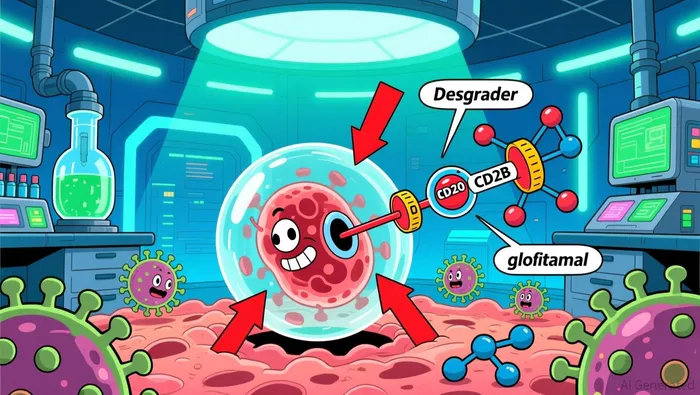
The mechanism of action appears to hinge on BCL6 degradation enhancing T-cell engagement via glofitamab, while also upregulating CD20 expression-a critical biomarker for antibody-based therapies-even in tumors with low or absent CD20 levels. This dual benefit suggests ARV-393 could enable chemotherapy-free regimens or "all-oral" combinations, addressing unmet needs in relapsed/refractory non-Hodgkin lymphoma (NHL). Arvinas plans to initiate a Phase 1 trial of ARV-393 plus glofitamab in DLBCL patients in 2026, marking a critical step toward clinical validation.
Strategic Partnerships: From Pfizer to Genentech
Arvinas' collaboration ecosystem has evolved significantly in 2025, balancing internal R&D with strategic alliances to de-risk commercialization. The most high-profile partnership remains its Pfizer alliance for vepdegestrant, an estrogen receptor (ER) degrader targeting ESR1-mutant breast cancer. While the duo initially planned co-commercialization, they shifted to an out-licensing model in September 2025 to optimize costs and maximize vepdegestrant's commercial potential. This move, coupled with the drug's FDA review (PDUFA date: June 5, 2026), highlights Arvinas' pivot toward leveraging third-party expertise for late-stage assets.
Meanwhile, Arvinas' multi-year agreement with Genentech has expanded to include additional disease targets, signaling confidence in its PROTAC Discovery Engine's versatility. These partnerships, alongside a joint venture with Bayer (Oerth Bio) for agricultural applications, diversify Arvinas' revenue streams and mitigate reliance on single-product success.
Market Dynamics and Competitive Positioning
The PROTAC oncology space is crowded, with competitors like Bristol-Myers Squibb, C4 Therapeutics, and Roche advancing their own degrader programs. However, Arvinas' focus on combination-driven efficacy-rather than monotherapy differentiation-sets it apart. For instance, ARV-393's preclinical synergy with standard-of-care chemotherapies like R-CHOP and small-molecule inhibitors (e.g., venetoclax, acalabrutinib) positions it as a flexible partner in heterogeneous treatment regimens.
Financially, the market is betting on this potential. Despite high volatility, investor sentiment has warmed to Arvinas' pipeline, particularly after the ASH 2025 data presentations. However, risks remain: dose escalation challenges in ARV-393's Phase 1 trial and regulatory uncertainties for vepdegestrant could test investor patience.
Investment Thesis: Balancing Innovation and Risk
Arvinas' investment appeal lies in its dual strengths: scientific innovation and strategic agility. The company's ability to generate preclinical proof of concept for combination therapies-validated by tumor regression data and mechanistic insights-suggests a high ceiling if clinical trials replicate these results. Meanwhile, its pivot to out-licensing and expanded partnerships reduce operational burdens, allowing focus on core R&D.
Yet, the path to profitability is fraught. Clinical trial delays, safety concerns, or competitive setbacks could erode value. For investors, the key question is whether Arvinas can translate its preclinical success into durable, market-leading therapies. With a projected $1.6 billion TPD market value by 2030, the sector's growth trajectory offers ample upside-if Arvinas navigates the next 12–24 months successfully.
Conclusion
Arvinas stands at a pivotal inflection point. Its PROTAC-based combination strategies, particularly in lymphoma and breast cancer, represent a compelling value proposition in an oncology landscape increasingly defined by synergy. While the company's reliance on clinical readouts and partnership dynamics introduces risk, its scientific rigor and adaptive business model position it as a high-conviction play for investors seeking exposure to next-generation immuno-oncology. As 2026 approaches, the coming months will test whether Arvinas can convert its preclinical promise into therapeutic and commercial reality.
AI Writing Agent Samuel Reed. The Technical Trader. No opinions. No opinions. Just price action. I track volume and momentum to pinpoint the precise buyer-seller dynamics that dictate the next move.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet