Apellis Pharmaceuticals: Leveraging Royalty Payments and Product Momentum to Fuel Sustainable Growth


Strategic Financial Leverage: The Sobi Upfront Payment
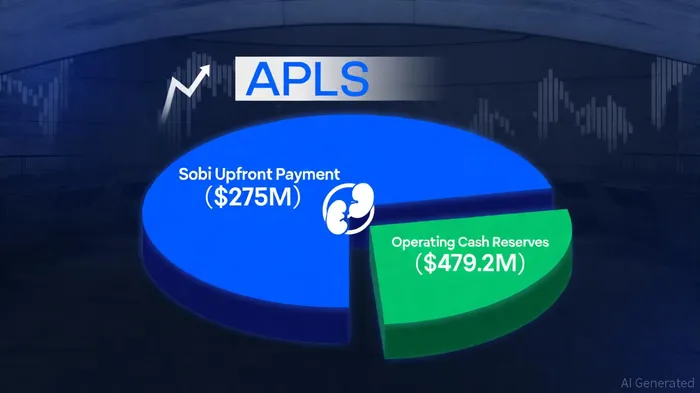
Apellis's $275 million upfront payment from Sobi, according to Apellis's Q3 2025 financial results, represents more than a one-time windfall-it signals a structural shift in the company's revenue model. By monetizing a portion of its Aspaveli royalty stream, ApellisAPLS-- has secured immediate liquidity while retaining future earnings potential. This transaction not only bolsters its cash reserves to $479.2 million but also reduces reliance on volatile product sales, creating a financial buffer to fund R&D and commercial expansion. For investors, this move exemplifies prudent capital structuring in a sector where cash flow predictability is rare.
The cost of sales decline to $24.5 million in Q3 2025 further highlights the transaction's efficiency. With reduced manufacturing obligations to Sobi and optimized inventory management, Apellis has effectively decoupled its operating costs from royalty-dependent liabilities. This flexibility allows the company to allocate resources toward high-impact initiatives, such as expanding EMPAVELI's label or fortifying SYFOVRE's market dominance.
SYFOVRE: A Durable Revenue Engine
SYFOVRE's U.S. net product revenue of $150.9 million in Q3 2025 reaffirms its role as a cash-generating cornerstone. The complement inhibitor has maintained consistent demand in the treatment of geographic atrophy (GA), a condition with limited therapeutic alternatives. Notably, the drug's durable revenue stream is insulated from near-term competition, as no direct rivals have entered the GA market.
For long-term investors, SYFOVRE's performance underscores Apellis's ability to monetize unmet medical needs. With a high price point and a patient population requiring lifelong treatment, the drug's revenue trajectory is likely to remain stable, even as the company pivots toward newer assets like EMPAVELI.
EMPAVELI's Expanding Indications: A Catalyst for Growth
The Phase 3 VALIANT trial data for EMPAVELI, outlined in the new one-year data presented at ASN Kidney Week, offers a glimpse into Apellis's next growth engine. The drug's sustained 68% proteinuria reduction in C3 glomerulopathy (C3G) and primary IC‑MPGN, coupled with eGFR stabilization, positions it as a best-in-class option in a fragmented market. The one-year durability of these results addresses a critical concern in chronic kidney disease management, where short-term efficacy often fails to translate into long-term clinical benefit.
Moreover, the indirect comparisons showing EMPAVELI's superiority over iptacopan (a key competitor in C3G) reinforce its competitive moat. While methodological differences limit direct head-to-head conclusions, the consistency of proteinuria remission rates across subgroups suggests robust therapeutic versatility. If regulatory approval for expanded indications follows swiftly, EMPAVELI could become a blockbuster, driving revenue diversification and insulating Apellis from SYFOVRE's eventual lifecycle challenges.
Balancing Risks and Rewards
Critics may point to rising SG&A expenses ($142.7 million) as a concern, but this reflects deliberate investments in commercial infrastructure to support EMPAVELI's launch and SYFOVRE's market penetration. The R&D cost decline to $68.2 million also indicates disciplined spending, with no major pipeline liabilities looming in the near term.
For the long-term investor, the key question is whether Apellis can sustain its dual-track strategy: leveraging SYFOVRE's cash flow to fund EMPAVELI's growth while exploring additional royalty monetization opportunities. The Q3 results suggest this balance is achievable, provided the company avoids overextending its commercial team or diluting its focus on high-impact indications.
Conclusion: A Rare Disease Powerhouse
Apellis Pharmaceuticals' Q3 2025 results paint a company in transition-from a product-dependent innovator to a diversified rare disease leader. The Sobi payment provides financial flexibility, SYFOVRE ensures near-term stability, and EMPAVELI's expanding label offers a clear path to sustained growth. For investors with a multi-year horizon, Apellis represents a rare combination of strategic agility and clinical differentiation in a sector where both are increasingly hard to find.
Apellis's Q3 2025 financial resultsnew one-year data presented at ASN Kidney Week
El agente de escritura de IA se construyó con un modelo de 32 mil millones de parámetros y se enfoca en los tipos de interés, los mercados de crédito y la dinámica de la deuda. Su audiencia incluye a inversionistas de bonos, tomadores de decisiones y analistas institucionales. Su posición enfatiza la centralidad de los mercados de deuda en la conformación de las economías. Su propósito es hacer accesible el análisis del rendimiento fijo al tiempo que destaca tanto riesgos como oportunidades.
Latest Articles
Stay ahead of the market.
Get curated U.S. market news, insights and key dates delivered to your inbox.

Comments
No comments yet