Zoetis Solidifies First-Mover Advantage in Canine Pain Management with Regulatory and Clinical Gains
The veterinary biologics market is undergoing a transformative shift, with monoclonal antibodies (mAbs) redefining standards for chronic disease management in companion animals. At the forefront of this revolution is ZoetisZTS--, whose strategic advancements in canine osteoarthritis (OA) pain therapies have cemented its first-mover advantage. By securing regulatory approvals, updating product labels with real-world data, and publishing robust clinical evidence, Zoetis has positioned itself as the dominant player in a rapidly expanding niche.
Regulatory Momentum and Label Enhancements
Zoetis' flagship OA therapy, Librela (bedinvetmab), has been a cornerstone of its innovation strategy. Since its U.S. launch in October 2023, the company has leveraged post-approval data to refine its product offering. In February 2025, Zoetis submitted a label supplement to the FDA, incorporating real-world safety and efficacy insights, according to MarketBeat. The updated label includes a client information sheet and revised guidance for veterinarians, reflecting a commitment to transparency and user education, as reported by DVM360. Crucially, adverse events reported during this period remained consistent with FDA expectations, underscoring the drug's safety profile, as noted in a Zoetis press release.
Parallel progress in Europe further strengthens Zoetis' global footprint. The European Medicines Agency's Committee for Veterinary Medicinal Products (CVMP) recently issued a positive opinion for Lenivia (izenivetmab), a three-month-acting mAb for OA pain in dogs, according to Business Wire. If approved, Lenivia would offer a significant leap in convenience for pet owners, aligning with the industry's shift toward long-acting therapies.
Clinical Validation and Competitive Edge
Zoetis has also prioritized head-to-head studies to differentiate its offerings. A March 2025 clinical trial published in Frontiers in Veterinary Science directly compared Librela to meloxicam, a widely used nonsteroidal anti-inflammatory drug (NSAID). The study found Librela to be equally effective in pain reduction, with just four adverse events versus 17 for meloxicam, according to a DVM360 article. This data not only reinforces Librela's role as a first-line therapy but also addresses a critical unmet need: reducing the gastrointestinal and renal risks associated with long-term NSAID use, as discussed in an NCBI review.
A Sparse Competitive Landscape
The lack of viable alternatives amplifies Zoetis' dominance. While competitors like Elanco and Boehringer Ingelheim are exploring mAb applications in veterinary oncology and dermatology, the OA pain segment remains underserved outside Zoetis' portfolio, according to a LinkedIn post. Market research indicates that Librela, along with other Zoetis mAbs like Cytopoint and Solensia, accounted for 72.64% of the 2024 veterinary mAb market, per Mordor Intelligence. This leadership is attributed to Zoetis' early adoption of mAb technology, streamlined dosing regimens, and strong brand recognition.
Emerging competitors, such as Exubrion Therapeutics and Elenagen, are developing novel biologics like Synovetin OA (radiosynoviorthesis) and SQSTM1/p62 plasmid therapy, as reported in a PR Newswire release. However, these therapies are still in early clinical stages and face regulatory hurdles. For now, Zoetis' established pipeline and real-world evidence provide a formidable barrier to entry.
Market Projections and Investment Implications
The veterinary mAb market is forecasted to grow at a compound annual rate of 16.61% from 2025 to 2030, driven by rising pet ownership and demand for premium care, according to Data Bridge Market Research. Zoetis' dual focus on regulatory agility and clinical differentiation positions it to capture a disproportionate share of this growth. With Librela and Lenivia addressing both U.S. and European markets, the company is uniquely poised to monetize its first-mover status while competitors remain in development.
For investors, Zoetis' strategic execution in canine pain management exemplifies the power of combining innovation with regulatory foresight. As the market evolves, the company's ability to translate scientific advances into commercial success will likely remain a key driver of long-term value.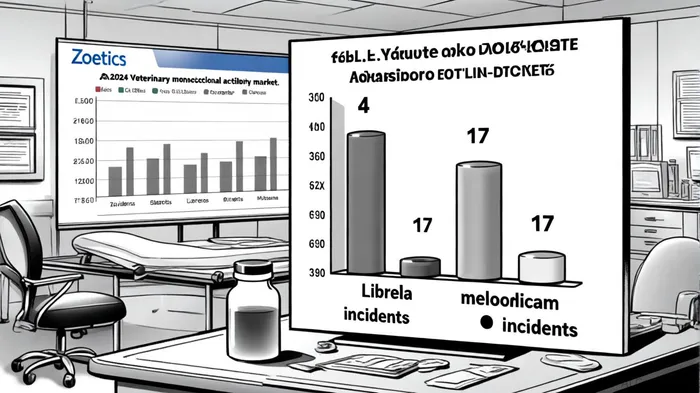

Comentarios
Aún no hay comentarios