Xencor's Bispecific Antibody Platform: Strategic and Financial Implications of Q4 2025 Data Readouts
In the rapidly evolving landscape of bispecific antibody development, XencorXNCR-- Inc. (XNCR) has positioned itself as a key player leveraging its XmAb platform to engineer next-generation therapeutics. As the biotech sector grapples with the dual challenges of clinical validation and financial sustainability, Xencor's Q4 2025 updates offer a compelling case study of strategic realignment and partnership-driven growth. This analysis evaluates the company's progress, focusing on the implications of its data readouts and financial positioning.
Clinical Pipeline: Progress and Risks
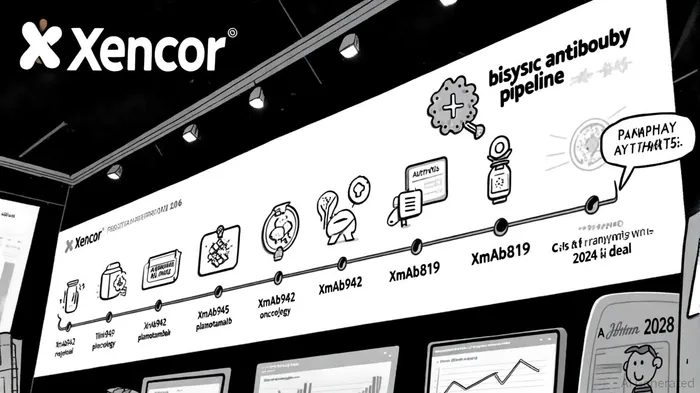
Xencor's bispecific antibody programs are advancing across both oncology and autoimmune disease indications. The most notable near-term catalyst is XmAb942, an extended half-life anti-TL1A antibody for inflammatory bowel disease (IBD). According to a report by Xencor's investor relations team, XmAb942 demonstrated a half-life of over 71 days in healthy volunteers, supporting a 12-week maintenance dosing interval[1]. This pharmacokinetic profile positions it as a potential best-in-class candidate for ulcerative colitis (UC), with the XENITH-UC Phase 2b trial set to enroll 220 patients and evaluate clinical remission by week 12[1].
In oncology, XmAb819 (ENPP3 x CD3), a bispecific T-cell engager for clear cell renal cell carcinoma (ccRCC), is in Phase 1 dose escalation. Early data show no maximum tolerated dose reached, with RECIST responses observed[1]. Meanwhile, plamotamab (CD20 x CD3), partnered with Johnson & Johnson under a $1.3 billion deal[3], is advancing to Phase 1b/2a trials for B-cell malignancies. These programs underscore Xencor's ability to generate clinical momentum, though setbacks like Roche's discontinuation of its IL-15 bispecific (which used Xencor's Fc technology)[3] highlight the inherent risks in bispecific development.
Financial Resilience and Partnership Dynamics
Xencor's financial health is bolstered by a combination of upfront payments, milestone-driven revenue, and strategic partnerships. As stated by the company in its Q4 2024 financial report, Xencor ended the period with $706.7 million in cash and equivalents, with a projected runway extending into 2028[1]. This stability is partly attributable to milestone payments from AmgenAMGN-- ($30 million) and NovartisNVS-- ($4 million)[1], as well as the J&J collaboration for plamotamab[3].
The J&J partnership, in particular, represents a strategic pivot. By licensing plamotamab, Xencor secures not only financial support but also access to J&J's global commercial infrastructure, a critical asset for bispecifics—a class still unproven in the market. This move aligns with Xencor's broader strategy of narrowing its pipeline to focus on core protein engineering strengths[1], reducing R&D burn while maintaining exposure to high-potential programs.
Strategic Implications and Market Positioning
The bispecific antibody market remains fragmented, with limited approved therapies and high attrition rates. Xencor's XmAb platform, which enables simpler IgG-based bispecific formats with enhanced potency[1], offers a differentiated approach. However, the discontinuation of Roche's IL-15 bispecific[3] serves as a cautionary tale: even with platform expertise, clinical and partnership risks persist.
From a market positioning perspective, Xencor's focus on autoimmune diseases (e.g., UC, rheumatoid arthritis) and oncology (e.g., ccRCC, prostate cancer) targets areas with unmet needs and high pricing potential. The XENITH-UC trial, if successful, could establish XmAb942 as a first-line therapy for IBD, a $10 billion market[1]. Meanwhile, XmAb819's progress in ccRCC—a niche but high-margin oncology segment—further diversifies Xencor's value proposition.
Conclusion: Balancing Optimism and Caution
Xencor's Q4 2025 updates reflect a company in transition. The clinical advancements in XmAb942 and XmAb819, coupled with the J&J partnership, provide a strong foundation for near-term growth. Financially, the company's cash runway and milestone-driven revenue model offer insulation against short-term volatility. However, the bispecific space is inherently risky, and setbacks like Roche's IL-15 exit[3] underscore the need for diversified pipelines.
For investors, Xencor presents a high-conviction opportunity: a platform with technical differentiation, a narrowing but focused pipeline, and a capital structure that supports key inflection points. Yet, the path to commercialization remains uncertain, and success will hinge on the XENITH-UC trial and other 2025 data readouts. As the biotech sector braces for a wave of bispecific approvals or failures, Xencor's ability to navigate this landscape will define its long-term value.

Comentarios
Aún no hay comentarios