Wegovy’s Cardiovascular Edge: How Novo Nordisk is Redefining the GLP-1 Market
The obesity and diabetes drug market has become a battleground for biotech giants, with NovoNVO-- Nordisk’s Wegovy (semaglutide) and Eli Lilly’s tirzepatide leading the charge. While tirzepatide has shown superior weight loss efficacy in clinical trials, Wegovy’s cardiovascular benefits have emerged as a critical differentiator, enabling Novo NordiskNVO-- to carve out a unique value proposition in the GLP-1 receptor agonist (GLP-1 RA) space. This strategic positioning, combined with real-world evidence and regulatory expansions, underscores Novo’s long-term vision to dominate the obesity-drug sector.
The Weight Loss vs. Cardiovascular Dilemma
In the 2025 head-to-head trial SURMOUNT-5, tirzepatide outperformed Wegovy in weight loss, with participants losing 20.2% of their body weight compared to 13.7% for Wegovy over 72 weeks [2]. A meta-analysis further confirmed this trend, showing tirzepatide’s mean weight loss advantage of 4.23 kg over semaglutide [3]. However, weight loss alone is not the sole metric driving market dynamics. Cardiovascular outcomes—particularly in patients with preexisting conditions—have become a decisive factor for both investors and healthcare providers.
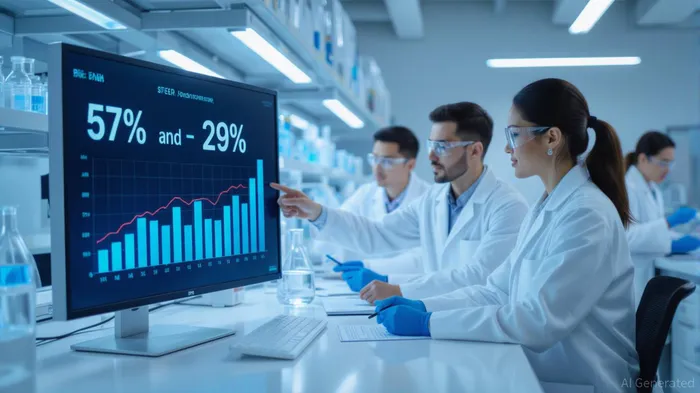
Here, Wegovy shines. The STEER real-world study revealed that Wegovy reduced the risk of heart attack, stroke, or death by 57% compared to tirzepatide in patients with overweight/obesity and cardiovascular disease (CVD). Even when accounting for treatment gaps, Wegovy still demonstrated a 29% risk reduction [1]. These findings align with Novo’s earlier SELECT and SCORE trials, which showed Wegovy’s ability to cut major adverse cardiovascular events (MACE) by 20–57% [1]. The semaglutide molecule appears to confer a cardiovascular advantage that tirzepatide lacks, at least in current data.
Strategic Differentiation: Novo Nordisk’s Playbook
Novo Nordisk has leveraged these cardiovascular benefits to position Wegovy as a “therapeutic necessity” rather than just a weight-loss tool. This strategy is paying off: despite manufacturing challenges and pricing pressures, Wegovy’s real-world cardiovascular data has allowed Novo to outpace competitors [1]. The company has further solidified its edge by expanding Wegovy’s indications. In 2025, the FDA approved Wegovy for treating noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) in adults with advanced liver fibrosis [2], broadening its appeal to hepatologists and gastroenterologists.
Novo’s long-term value creation also hinges on next-generation therapies. The company is advancing CagriSema, a combination of semaglutide and canagliflozin, which targets both GLP-1 and SGLT2 pathways. This dual-action approach could enhance cardiovascular benefits while maintaining weight loss, addressing a key limitation of current GLP-1 RAs [3]. Meanwhile, Eli Lilly’s tirzepatide is still in trials to determine whether it matches Wegovy’s cardiovascular profile [4], giving Novo a critical head start.
The Investment Thesis
For investors, the key takeaway is clear: Novo Nordisk has transformed Wegovy into a multifaceted therapeutic platform. While tirzepatide may attract patients seeking maximum weight loss, Wegovy’s cardiovascular advantages—backed by robust clinical and real-world data—position it as the preferred choice for high-risk populations. This dual focus on efficacy and safety creates a sustainable competitive moat, especially as obesity-related comorbidities drive demand for comprehensive treatments.
Moreover, Novo’s partnerships with insurers and investments in manufacturing scalability ensure that Wegovy remains accessible and profitable. The company’s recent $10 billion revenue milestone for Wegovy in 2025 underscores the financial viability of this strategy [1]. As the GLP-1 market grows, Novo’s emphasis on cardiovascular outcomes and regulatory expansions will likely cement its leadership, making it a compelling long-term investment.
Source:
[1] Novo Nordisk's Wegovy® cuts risk of heart attack, stroke or ... [https://www.prnewswire.com/news-releases/novo-nordisks-wegovy-cuts-risk-of-heart-attack-stroke-or-death-by-57-compared-to-tirzepatide-in-real-world-study-of-people-with-obesity-and-cardiovascular-disease-302542590.html][2] Novo Nordisk A/S: Wegovy® Approved in the U.S. for ... [https://www.scbio.org/novo-nordisk-a-s-wegovy-approved-in-the-u-s-for-the-treatment-of-mash/][3] Comparative Efficacy of Tirzepatide vs. Semaglutide in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12151102/][4] Semaglutide and Tirzepatide Lead the Way in ... [https://www.pharmacytimes.com/view/semaglutide-and-tirzepatide-lead-the-way-in-comprehensive-diabetes-and-obesity-management]

Comentarios
Aún no hay comentarios