Verastem Advances with Phase 3 Trial Progress and Product Launch.
PorAinvest
domingo, 17 de agosto de 2025, 3:27 am ET1 min de lectura
VSTM--
The study, which began in March 2024, is an open-label, randomized trial, allowing participants to switch to the new drugs if their disease progresses. This trial is crucial for exploring new treatment options for this challenging cancer, which has the potential to impact the competitive environment in oncology.
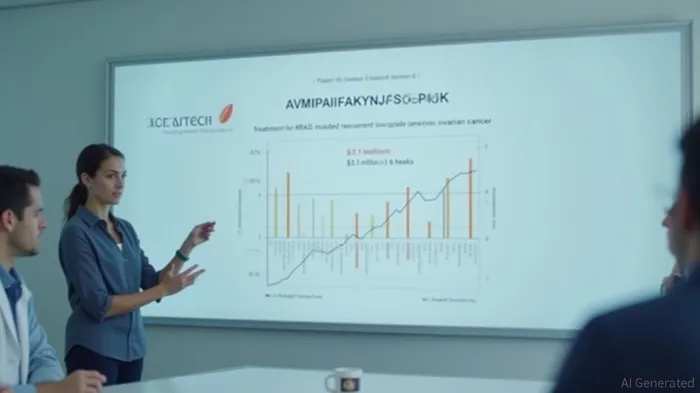
In addition to its progress in the Phase 3 trial, Verastem, Inc. also highlighted the FDA approval of its AVMAPKI FAKZYNJA CO-PACK, a treatment for KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC). The company received this approval nearly two months ahead of schedule, entering the market with the first-ever treatment for this specific type of cancer. The product has generated $2.1 million in net product revenue within just six weeks of its launch, indicating strong market reception.
Verastem, Inc. has a short float of 37.03%, suggesting elevated bearish bets that position the stock for significant gains if a bullish trigger were to materialize. While the company acknowledges the potential of VSTM as an investment, it believes certain AI stocks offer greater upside potential and carry less downside risk.
References:
[1] https://finance.yahoo.com/news/verastem-advances-phase-3-trial-072207039.html
[2] https://www.oncnursingnews.com/view/avutometinib-defactinib-shows-soc-potential-in-lg-serous-ovarian-cancer
[3] https://femtechinsider.com/abbvie-receives-uk-mhra-approval-for-ovarian-cancer-treatment-elahere/
Verastem, Inc. (NASDAQ:VSTM) is advancing with its Phase 3 ovarian cancer trial and AVMAPKI FAKZYNJA CO-PACK generating revenue. The company's focus on discovering and commercializing novel cancer therapies has led to the FDA approval of AVMAPKI FAKZYNJA CO-PACK, a treatment for KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC). The product has generated $2.1 million in net product revenue within six weeks of its launch. The company has a short float of 37.03%.
Verastem, Inc. (NASDAQ:VSTM), a development-stage biopharmaceutical company, has made significant strides in its efforts to combat ovarian cancer. On August 8, 2025, the company announced an update to its Phase 3 study in ovarian cancer, known as RAMP 301. This trial is designed to assess the safety and effectiveness of a new combination therapy for recurrent low-grade serous ovarian cancer (LGSOC) using the oral drugs avutometinib and defactinib against standard treatments.The study, which began in March 2024, is an open-label, randomized trial, allowing participants to switch to the new drugs if their disease progresses. This trial is crucial for exploring new treatment options for this challenging cancer, which has the potential to impact the competitive environment in oncology.
In addition to its progress in the Phase 3 trial, Verastem, Inc. also highlighted the FDA approval of its AVMAPKI FAKZYNJA CO-PACK, a treatment for KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC). The company received this approval nearly two months ahead of schedule, entering the market with the first-ever treatment for this specific type of cancer. The product has generated $2.1 million in net product revenue within just six weeks of its launch, indicating strong market reception.
Verastem, Inc. has a short float of 37.03%, suggesting elevated bearish bets that position the stock for significant gains if a bullish trigger were to materialize. While the company acknowledges the potential of VSTM as an investment, it believes certain AI stocks offer greater upside potential and carry less downside risk.
References:
[1] https://finance.yahoo.com/news/verastem-advances-phase-3-trial-072207039.html
[2] https://www.oncnursingnews.com/view/avutometinib-defactinib-shows-soc-potential-in-lg-serous-ovarian-cancer
[3] https://femtechinsider.com/abbvie-receives-uk-mhra-approval-for-ovarian-cancer-treatment-elahere/
Divulgación editorial y transparencia de la IA: Ainvest News utiliza tecnología avanzada de Modelos de Lenguaje Largo (LLM) para sintetizar y analizar datos de mercado en tiempo real. Para garantizar los más altos estándares de integridad, cada artículo se somete a un riguroso proceso de verificación con participación humana.
Mientras la IA asiste en el procesamiento de datos y la redacción inicial, un miembro editorial profesional de Ainvest revisa, verifica y aprueba de forma independiente todo el contenido para garantizar su precisión y cumplimiento con los estándares editoriales de Ainvest Fintech Inc. Esta supervisión humana está diseñada para mitigar las alucinaciones de la IA y garantizar el contexto financiero.
Advertencia sobre inversiones: Este contenido se proporciona únicamente con fines informativos y no constituye asesoramiento profesional de inversión, legal o financiero. Los mercados conllevan riesgos inherentes. Se recomienda a los usuarios que realicen una investigación independiente o consulten a un asesor financiero certificado antes de tomar cualquier decisión. Ainvest Fintech Inc. se exime de toda responsabilidad por las acciones tomadas con base en esta información. ¿Encontró un error? Reportar un problema

Comentarios
Aún no hay comentarios