Vaxart, Inc. (VXRT): Pioneering Oral Vaccines Amid Strategic and Financial Crossroads
In a crowded biotech landscape, Vaxart, Inc. (VXRT) has positioned itself as a disruptor in the oral vaccine market through its proprietary platform, which eliminates the need for refrigeration, needles, and complex delivery systems. At the H.C. Wainwright 27th Annual Global Investment Conference on September 8, 2025, the company underscored its progress in developing second-generation oral vaccines, particularly for norovirus, influenza, and HPV, while addressing critical questions about market access and financial sustainability.
Innovation: A Proprietary Platform with Mucosal Immunity Edge
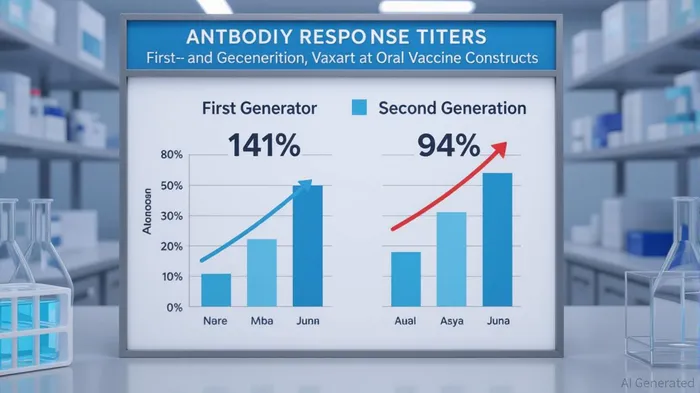
Vaxart's core innovation lies in its oral recombinant vaccine technology, which leverages a pill-based delivery system to stimulate both mucosal and systemic immunity. This approach is particularly advantageous for diseases like norovirus, where mucosal defenses are critical to preventing infection. Recent Phase 1 data revealed that second-generation norovirus constructs generated a 141% increase in GI.1 norovirus blocking antibody titers and a 94% increase in GII.4 titers compared to first-generation versions, correlating with enhanced protection against infection [2]. Fecal IgA responses—a key indicator of mucosal immunity—also surged by 25-fold for GII.4 and 10-fold for GI.1 after a single dose, reinforcing the platform's potential [4].
The company's pipeline extends beyond norovirus, with candidates targeting seasonal influenza and HPV. These programs highlight Vaxart's ambition to address unmet needs in infectious disease prevention, particularly in low-resource settings where cold-chain logistics and needle-based vaccines pose barriers [1].
Market Access: Partnerships and Regulatory Hurdles
Despite its technological promise, Vaxart's path to commercialization hinges on securing partnerships and regulatory approvals. In 2025, the company faced a setback when its BARDA-funded Phase 2b trial for an oral COVID-19 vaccine was halted by a stop-work order, effectively freezing enrollment while follow-up for existing participants continued [3]. This development underscores the volatility of relying on government contracts, though Vaxart remains optimistic about pursuing non-dilutive funding for its norovirus and influenza programs [5].
The company's financial runway is another critical factor. With a Q2 2025 net loss of $15.0 million and cash reserves expected to last until early 2026, Vaxart must secure partnerships or alternative funding to advance its Phase 2b trial for second-generation norovirus vaccines, slated for H2 2025 [4]. While larger competitors like ModernaMRNA-- and NovavaxNVAX-- boast R&D budgets exceeding $700 million annually, Vaxart's niche focus on oral delivery could attract partners seeking to diversify their vaccine portfolios [1].
Competitive Differentiation: Navigating a Crowded Field
Vaxart's primary competitors include industry giants such as PfizerPFE--, Johnson & Johnson, and SanofiSNY--, which dominate traditional vaccine markets. However, its oral platform offers a unique value proposition: needle-free administration, stability at room temperature, and mucosal immunity activation. These attributes align with global public health priorities, particularly in regions with limited healthcare infrastructure [5].
The company also differentiates itself through therapeutic vaccine candidates in immune-oncology, expanding its potential beyond infectious diseases. This dual focus on preventive and therapeutic applications could broaden its market access and investor appeal [1].
Conclusion: Balancing Innovation with Financial Realities
Vaxart's recent conference presentation highlighted its scientific progress and strategic vision, but the company's success will depend on overcoming financial and regulatory challenges. While its second-generation norovirus data is compelling, the path to commercialization requires robust partnerships and a clear regulatory strategy. For investors, Vaxart represents a high-risk, high-reward opportunity in the evolving oral vaccine market—a space where innovation and execution are equally critical.

Comentarios
Aún no hay comentarios