United Therapeutics' TETON-2 Trial: A Pivotal Step in Expanding Tyvaso's IPF Market Potential
United Therapeutics Corporation has made a transformative leap in its pursuit to expand the therapeutic and commercial footprint of Tyvaso (treprostinil) with the groundbreaking results of its TETON-2 trial for idiopathic pulmonary fibrosis (IPF). The trial, which evaluated nebulized Tyvaso in IPF patients, demonstrated a statistically significant 95.6 mL improvement in absolute forced vital capacity (FVC) from baseline to week 52 compared to placebo (p < 0.0001) [1]. This outcome not only underscores the drug's potential to address a critical unmet need in IPF but also positions United TherapeuticsUTHR-- to capitalize on a rapidly growing market.
Transformative Pipeline Progress: TETON-2 and Beyond
The TETON-2 trial's success marks a pivotal milestone for United Therapeutics. The 95.6 mL FVC improvement—a key metric for assessing lung function in IPF—was consistent across subgroups, including patients on background therapies like nintedanib or pirfenidone and those requiring supplemental oxygen [1]. Secondary endpoints, such as time to first clinical worsening and quality-of-life metrics (e.g., K-BILD questionnaire), also showed statistically significant benefits [1]. While endpoints like time to first acute exacerbation and overall survival did not reach statistical significance, they exhibited trends favoring Tyvaso, suggesting broader therapeutic potential [1].
The company is now poised to leverage these results, along with data from the ongoing TETON-1 trial (top-line results expected in mid-2026), to file a supplemental New Drug Application (sNDA) with the FDA for an IPF indication [1]. United Therapeutics has also signaled its intent to engage with the FDA by year-end 2025 to discuss expedited regulatory pathways, potentially accelerating approval timelines [1]. If successful, this would make Tyvaso the first inhaled therapy approved for IPF, offering a novel mechanism of action alongside the current antifibrotic standards of care.
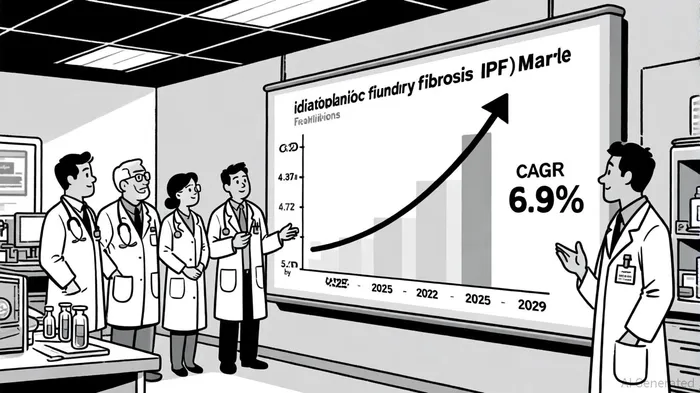
Market Expansion Potential: A $5.72 Billion Opportunity by 2029
The IPF market is a high-growth segment, projected to expand from USD 4.37 billion in 2025 to USD 5.72 billion by 2029, with a compound annual growth rate (CAGR) of 6.9% [2]. This growth is driven by an aging population, increased awareness of IPF, and the entry of innovative therapies. Currently, pirfenidone (Roche's Esbriet) and nintedanib (Boehringer Ingelheim's Ofev) dominate the market, with pirfenidone capturing 43.91% of market share in 2024 [2]. However, nintedanib is expected to grow at a faster CAGR of 8.0%, fueled by its broader label and favorable safety profile [2].
United Therapeutics' entry into this market with Tyvaso could disrupt the status quo. Inhaled therapies, including treprostinil, are gaining traction due to their localized delivery, which minimizes systemic side effects and improves tolerability—critical advantages for patients struggling with oral medications [2]. The inhalation segment is projected to grow at a 10.21% CAGR, outpacing the overall IPF market [2]. With over 100,000 IPF patients in the U.S. alone, the commercial potential for Tyvaso is substantial, particularly if it is approved as an adjunct to existing antifibrotic therapies [1].
Competitive Landscape: Navigating Emerging Threats and Opportunities
While United Therapeutics is making strides, the IPF pipeline is becoming increasingly competitive. Nerandomilast, Boehringer Ingelheim's oral PDE4B inhibitor, has shown promise in phase 3 trials (FIBRONEER-ILD), slowing lung function decline and potentially becoming the first novel IPF therapy in over a decade [3]. However, Tyvaso's unique inhaled formulation and demonstrated FVC improvement position it as a complementary option, particularly for patients who cannot tolerate oral therapies or require additional lung function support [1].
Analysts from Jefferies have highlighted that Tyvaso could be used in combination with pirfenidone or nintedanib, broadening its market reach [1]. This dual-therapy strategy could differentiate Tyvaso in a market where monotherapy remains the standard. Furthermore, United Therapeutics' experience in managing chronic respiratory conditions (e.g., pulmonary arterial hypertension) provides a strong foundation for IPF patient engagement and adherence support.
Investment Implications: A High-Stakes Bet on Innovation
The TETON-2 results and the broader IPF market dynamics present a compelling case for investors. United Therapeutics' ability to secure an IPF indication for Tyvaso would not only diversify its revenue streams but also establish a leadership position in a high-growth therapeutic area. With the TETON-1 trial expected to deliver data in mid-2026, the company is on a trajectory to potentially reshape IPF treatment paradigms.
However, risks remain. Regulatory hurdles, competition from emerging therapies like nerandomilast, and payer reimbursement challenges could temper growth. That said, the demonstrated clinical efficacy of Tyvaso, coupled with United Therapeutics' aggressive regulatory strategy, suggests a strong likelihood of market penetration. For investors, the key will be monitoring the TETON-1 results and FDA interactions in late 2025, which could catalyze a re-rating of the company's valuation.

Comentarios
Aún no hay comentarios