Theraclion's MDR Certification: A Catalyst for Commercial Expansion and Investor Confidence
Theraclion's recent achievement of Medical Device Regulation (MDR) certification for its Sonovein® platform marks a pivotal turning point in the company's trajectory. This regulatory milestone, secured under the EU's stringent 2017/745 framework, not only validates the safety and efficacy of Sonovein® but also solidifies Theraclion's commercial positioning in Europe and beyond. According to a report by Business Wire, the certification confirms compliance with the EU's rigorous quality management standards, providing hospitals, physicians, and distributors with confidence in the long-term market viability of the device [1]. This is particularly significant given the transition from the older CE Mark certification, which Sonovein® held since 2019 but now must meet the higher bar of MDR requirements [2].
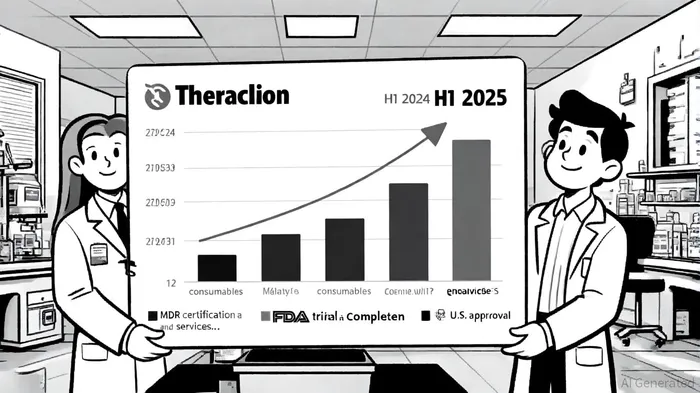
The implications of this certification extend beyond regulatory compliance. For investors, it represents a de-risking of Theraclion's European market access, which accounts for a substantial portion of its current revenue. Data from Yahoo Finance indicates that the company's first-half 2025 revenue surged 89% year-over-year to €835,000, driven by a 30% increase in consumables sales and a 232% rise in service revenue [3]. These recurring revenue streams, tied to the adoption of Sonovein® in treatment centers, suggest growing acceptance of the platform. The MDR certification further enhances this momentum by ensuring continuity in regulatory alignment, which is critical for maintaining partnerships and expanding into new markets.
Geographically, Theraclion has leveraged the certification to accelerate its expansion. The company opened new treatment centers in Bulgaria and Spain in 2025 and secured product placement contracts in these regions [4]. Such moves underscore a strategic focus on scaling operations in markets where Sonovein® has demonstrated clinical value. The MDR certification, by reinforcing the device's credibility, likely played a role in attracting these partnerships. Additionally, the appointment of a new Chief Commercial Officer in May 2025 signals an intensified push into the Middle East and Europe, where regulatory clarity is now a key differentiator [5].
While Europe remains the core market, the MDR certification also indirectly supports Theraclion's U.S. ambitions. Sonovein® is currently limited to investigational use in the U.S., but the completion of a pivotal FDA clinical trial in June 2025—on schedule—has positioned the company for a potential breakthrough. As stated by Business Wire, the 12-month follow-up phase of the trial was finalized, with results expected in September 2025 [6]. A positive outcome could pave the way for an FDA marketing authorization application in Fall 2025, with approval anticipated by Q2 2026 [7]. The MDR certification, by demonstrating global regulatory rigor, may bolster the FDA's confidence in the platform's safety profile, indirectly enhancing the likelihood of U.S. approval.
Investor sentiment has already begun to reflect these developments. Morningstar notes that Theraclion's strategic hires, clinical progress, and revenue growth have collectively driven a surge in market confidence [8]. The company's ability to generate recurring revenue—particularly from consumables and services—has proven attractive to investors seeking sustainable growth. With the MDR certification now in place, the risk of regulatory disruption in Europe is mitigated, allowing investors to focus on the company's expansion potential.
However, challenges remain. The U.S. market, though lucrative, is still out of reach until FDA approval is secured. Additionally, competition in the venous disease treatment sector is intensifying, with larger players investing in similar technologies. Theraclion's success will depend on its ability to maintain its first-mover advantage in Europe while navigating the complexities of U.S. regulatory and commercial landscapes.
In conclusion, Theraclion's MDR certification for Sonovein® is more than a regulatory checkbox—it is a strategic enabler of commercial growth and investor trust. By aligning with the EU's most stringent standards, the company has reinforced its market credibility, secured recurring revenue streams, and laid the groundwork for global expansion. For investors, this milestone, combined with robust financial performance and a clear path to U.S. approval, presents a compelling case for long-term value creation.



Comentarios
Aún no hay comentarios