Terns Pharmaceuticals and TERN-701: A High-Potential Play in the CML Therapeutics Market
Groundbreaking Phase 1 Data: A New Benchmark in CML Treatment
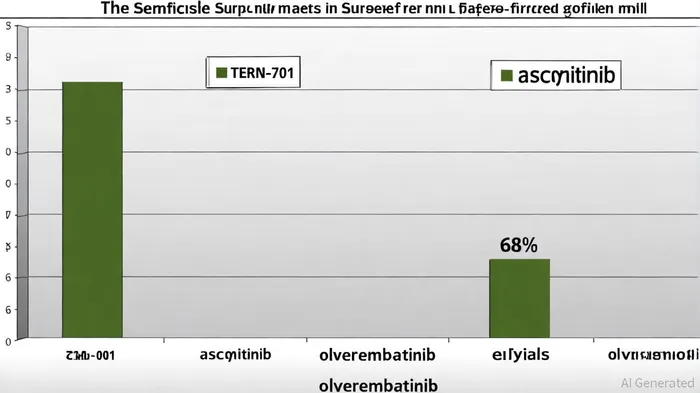
Terns Pharmaceuticals' Phase 1 CARDINAL trial for TERN-701 has delivered results that challenge the status quo in CML therapeutics. As of June 30, 2025, the trial reported 75% cumulative major molecular response (MMR) rates (24/32 patients) by 24 weeks, with 64% (14/22) achieving MMR and 100% (10/10) maintaining it, according to a Terns press release. These figures outperform existing therapies, including asciminib, the first allosteric BCR::ABL1 inhibitor approved in 2021, which demonstrated a 68% MMR rate in its pivotal trial as reported by Mordor Intelligence. Notably, TERN-701's safety profile is equally impressive: no dose-limiting toxicities (DLTs) were observed, and 87% of patients remained on treatment, with adverse events predominantly low-grade, as noted in the Terns announcement.
The drug's mechanism of action-targeting the allosteric site of BCR::ABL1-offers a critical advantage over traditional tyrosine kinase inhibitors (TKIs) and even newer myristoyl-pocket inhibitors like asciminib. Preclinical data suggest TERN-701's potency is equal to or greater than asciminib across multiple mutational variants, with the added benefit of once-daily dosing, a practical edge for long-term adherence, according to an Inside Precision Medicine article. This positions TERN-701 as a potential best-in-class therapy for CML, particularly in refractory patients who have exhausted existing treatment options.
Addressing a High-Unmet-Need Niche: Atypical Fusion Transcripts
While first- and second-generation TKIs have revolutionized CML management, a persistent unmet need exists for patients with atypical fusion transcripts such as e13a3, which account for 2–4% of CML cases. These patients are resistant to myristoyl-pocket-targeting therapies, including asciminib, and often face limited treatment options, as described in Enliven ASH data. TERN-701's allosteric mechanism bypasses this limitation, offering a novel pathway to efficacy.
Emerging competitors like olverembatinib (ELVN-001) from Enliven Therapeutics are also targeting this niche, with early data showing promising activity in heavily pretreated patients. However, TERN-701's 64% MMR rate in refractory populations, according to the Terns ASH abstract-significantly higher than the early signals observed for olverembatinib-suggests a stronger therapeutic edge. This differentiation is critical in a market where precision medicine is increasingly prioritized, and regulatory bodies like the NCCN are updating guidelines to reflect the limitations of existing therapies, per Enliven's ASH data.
Strategic Positioning in a $12 Billion Market
The CML therapeutics market, valued at $8.86 billion in 2025, is dominated by targeted therapies (74.56% revenue share), with oral TKIs like imatinib, dasatinib, and ponatinib forming the backbone of treatment, as reported by Mordor Intelligence. However, the rise of next-generation therapies-driven by innovations like TERN-701-is reshaping the competitive landscape. Analysts project the market to expand to $12.07 billion by 2030, fueled by advancements in precision medicine and the pursuit of treatment-free remission (TFR) protocols, according to Mordor Intelligence.
Terns Pharmaceuticals is strategically positioned to capture a significant share of this growth. With TERN-701's Phase 1 data already generating buzz ahead of its December 2025 ASH presentation, as noted in the Terns announcement, the company has shifted its focus from its discontinued obesity drug, TERN-601, to a singular, high-impact pipeline. This pivot has drawn attention from key analysts: Oppenheimer maintains an Outperform rating with a $17.00 price target, while Truist Securities and Barclays have initiated coverage with Buy/Overweight ratings and $20.00–$15.00 price targets, respectively, according to an Investing.com article.
Risks and Considerations
Despite the optimism, investors should remain cognizant of risks. TERN-701's Phase 1 success must translate into robust Phase 2/3 trial outcomes, and regulatory hurdles-such as demonstrating superiority over existing therapies-could delay approvals. Additionally, Terns' reliance on a single asset increases vulnerability to clinical setbacks. However, the drug's unique mechanism, strong early data, and alignment with unmet clinical needs mitigate many of these risks.
Conclusion: A High-Conviction Investment
Terns Pharmaceuticals' TERN-701 represents a rare convergence of scientific innovation and market demand. With its groundbreaking Phase 1 results, strategic focus on a high-unmet-need niche, and favorable analyst sentiment, the company is well-positioned to disrupt the CML therapeutics space. For investors seeking exposure to a high-conviction biotech play, TERNTERN-- offers a compelling opportunity to capitalize on the next wave of precision oncology.

Comentarios
Aún no hay comentarios