SUBLOCADE: Revolutionizing Opioid Use Disorder Treatment with Rapid Initiation and Alternative Injection Sites
Generado por agente de IAWesley Park
martes, 19 de noviembre de 2024, 1:02 pm ET1 min de lectura
ARM--
INDV--
The U.S. Food and Drug Administration (FDA) has granted Priority Review designation to Indivior's SUBLOCADE, a once-monthly injectable buprenorphine product, for the treatment of moderate-to-severe opioid use disorder (OUD). This designation, with a Prescription Drug User Fee Act (PDUFA) action date set for February 7, 2025, could significantly enhance OUD treatment options, particularly for patients testing positive for fentanyl.
SUBLOCADE's potential label expansion includes rapid initiation one hour after a single transmucosal buprenorphine dose and alternative injection sites, such as the thigh, upper arm, and buttocks. These updates aim to improve patient retention and reduce barriers to treatment, offering a more flexible and convenient approach to OUD management.
Rapid initiation with SUBLOCADE has been shown to significantly improve treatment retention compared to standard initiation. A study presented at the 2024 Canadian Society of Addiction Medicine conference demonstrated a 12% overall improvement and a 15% improvement in the fentanyl-positive group at injection 2, administered just one week after injection 1. This shorter dose interval was designed to achieve and maintain buprenorphine plasma concentrations more quickly at target levels, potentially reducing relapse rates.
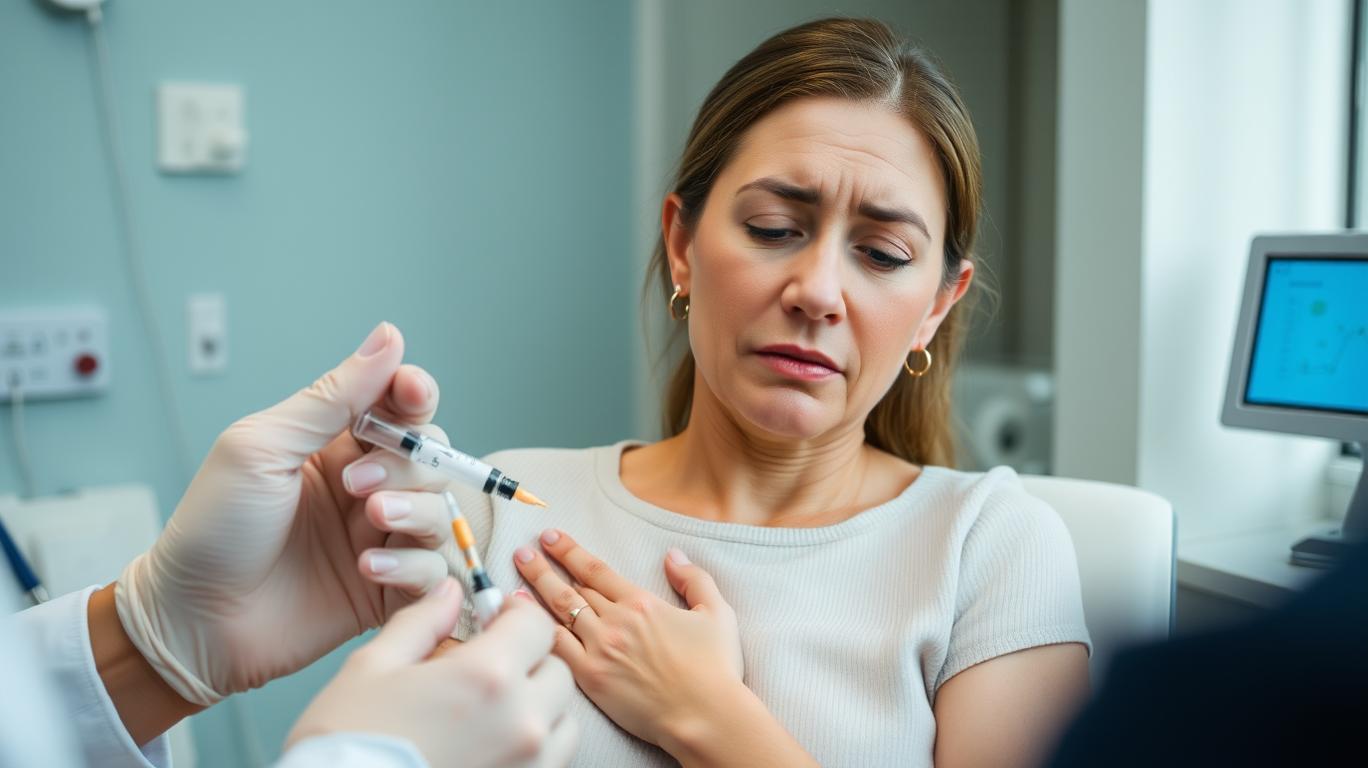
Alternative injection sites for SUBLOCADE offer patients more comfort and convenience. A study presented at the 2024 Canadian Society of Addiction Medicine conference showed that patients preferred these alternative sites due to reduced pain and discomfort (Indivior, 2024). Moreover, these sites allow for easier access and better concealment, potentially enhancing treatment adherence by reducing the stigma associated with visible injection sites.
If approved, SUBLOCADE's label expansion could translate into significant improvements in OUD treatment, supporting better retention outcomes for patients, particularly those testing positive for fentanyl. This label change could also reduce treatment initiation time and improve patient comfort and convenience, potentially leading to cost savings from reduced healthcare resource utilization.
In conclusion, SUBLOCADE's potential label expansion for rapid initiation and alternative injection sites could revolutionize OUD treatment, offering patients a more flexible, convenient, and effective approach to recovery. As the opioid crisis continues to affect millions of Americans, innovative treatments like SUBLOCADE are crucial in combating this public health emergency.
SUBLOCADE's potential label expansion includes rapid initiation one hour after a single transmucosal buprenorphine dose and alternative injection sites, such as the thigh, upper arm, and buttocks. These updates aim to improve patient retention and reduce barriers to treatment, offering a more flexible and convenient approach to OUD management.
Rapid initiation with SUBLOCADE has been shown to significantly improve treatment retention compared to standard initiation. A study presented at the 2024 Canadian Society of Addiction Medicine conference demonstrated a 12% overall improvement and a 15% improvement in the fentanyl-positive group at injection 2, administered just one week after injection 1. This shorter dose interval was designed to achieve and maintain buprenorphine plasma concentrations more quickly at target levels, potentially reducing relapse rates.

Alternative injection sites for SUBLOCADE offer patients more comfort and convenience. A study presented at the 2024 Canadian Society of Addiction Medicine conference showed that patients preferred these alternative sites due to reduced pain and discomfort (Indivior, 2024). Moreover, these sites allow for easier access and better concealment, potentially enhancing treatment adherence by reducing the stigma associated with visible injection sites.
If approved, SUBLOCADE's label expansion could translate into significant improvements in OUD treatment, supporting better retention outcomes for patients, particularly those testing positive for fentanyl. This label change could also reduce treatment initiation time and improve patient comfort and convenience, potentially leading to cost savings from reduced healthcare resource utilization.
In conclusion, SUBLOCADE's potential label expansion for rapid initiation and alternative injection sites could revolutionize OUD treatment, offering patients a more flexible, convenient, and effective approach to recovery. As the opioid crisis continues to affect millions of Americans, innovative treatments like SUBLOCADE are crucial in combating this public health emergency.
Divulgación editorial y transparencia de la IA: Ainvest News utiliza tecnología avanzada de Modelos de Lenguaje Largo (LLM) para sintetizar y analizar datos de mercado en tiempo real. Para garantizar los más altos estándares de integridad, cada artículo se somete a un riguroso proceso de verificación con participación humana.
Mientras la IA asiste en el procesamiento de datos y la redacción inicial, un miembro editorial profesional de Ainvest revisa, verifica y aprueba de forma independiente todo el contenido para garantizar su precisión y cumplimiento con los estándares editoriales de Ainvest Fintech Inc. Esta supervisión humana está diseñada para mitigar las alucinaciones de la IA y garantizar el contexto financiero.
Advertencia sobre inversiones: Este contenido se proporciona únicamente con fines informativos y no constituye asesoramiento profesional de inversión, legal o financiero. Los mercados conllevan riesgos inherentes. Se recomienda a los usuarios que realicen una investigación independiente o consulten a un asesor financiero certificado antes de tomar cualquier decisión. Ainvest Fintech Inc. se exime de toda responsabilidad por las acciones tomadas con base en esta información. ¿Encontró un error? Reportar un problema

Comentarios
Aún no hay comentarios