Strategic Expansion in Biopharma Partnerships: Evaluating the Long-Term Value Creation Potential of the FUJIFILM Biotechnologies and argenx Alliance
In the rapidly evolving biopharmaceutical landscape, strategic partnerships have become a cornerstone of innovation and scalability. The recent expansion of the allianceAENT-- between FUJIFILM Biotechnologies and argenx offers a compelling case study in how such collaborations can drive long-term value creation. By combining argenx's therapeutic expertise with FUJIFILM's cutting-edge modular manufacturing infrastructure, the partnership not only addresses immediate production needs for efgartigimod (marketed as VYVGART) but also aligns with broader industry trends toward supply chain resilience and localized production.
A Strategic Alliance for Scalability and Resilience
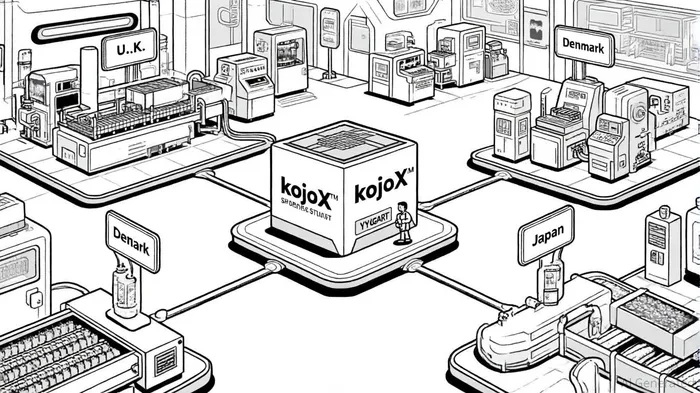
The core of this partnership lies in the construction of a U.S. manufacturing hub for efgartigimod at FUJIFILM's Holly Springs, North Carolina, site, set to begin operations in 2028. This facility, part of Phase II of the site's expansion, will add eight 20,000-liter mammalian cell culture bioreactors, doubling the site's capacity to 16 bioreactors [1]. Argentx, as the first tenant of this phase, gains access to FUJIFILM's kojoX™ global network, a modular and standardized system that enables “local-for-local” manufacturing. This approach reduces reliance on centralized production hubs, mitigating risks from geopolitical disruptions or logistical bottlenecks [2].
The kojoX network's flexibility is a critical differentiator. By standardizing processes across sites in the U.S., U.K., Denmark, and Japan, the system allows for rapid scaling and geographic diversification. For argenxARGX--, this means producing VYVGART for U.S. patients domestically while leveraging the same infrastructure to support international demand. As Lars Petersen, CEO of FUJIFILM Biotechnologies, noted, this partnership represents the first global end-to-end program utilizing the kojoX network, underscoring its potential to redefine supply chain strategies in the biopharma sector [3].
Market Dynamics and Therapeutic Potential
The financial rationale for this alliance is bolstered by the robust growth trajectory of efgartigimod. The global market for VYVGART was valued at $2.19 billion in 2024 and is projected to reach $5.50 billion by 2030, with a compound annual growth rate (CAGR) of 13.6% [4]. North America dominates this market, accounting for 89.52% of revenue in 2024, while the Asia-Pacific region is expected to emerge as a high-growth area. This expansion is driven by the drug's approval for two key indications—generalized myasthenia gravis (gMG) and chronic inflammatory demyelinating polyneuropathy (CIDP)—with gMG alone capturing 97.70% of 2024 revenues [4].
Moreover, the recent approval of a subcutaneous (SC) formulation of VYVGART in April 2025—a prefilled syringe for at-home administration—has further enhanced patient accessibility and adherence. Clinical trials have demonstrated its comparable safety and efficacy to intravenous (IV) infusions, positioning it as a disruptive force in autoimmune disease management [4].
Long-Term Value Creation: Beyond Manufacturing
The alliance's value extends beyond production. By securing early access to the kojoX network, argenx gains a strategic edge in scaling its pipeline. Efgartigimod's therapeutic versatility is already expanding, with ongoing trials targeting over 15 autoimmune diseases, including thyroid eye disease, primary Sjögren's syndrome, and lupus nephritis [4]. This broadens the drug's commercial potential and reduces reliance on a single indication.
For FUJIFILM, the partnership validates the kojoX model's scalability. The Holly Springs expansion, which will add $500 million in capital investment by 2028 [1], positions the company as a leader in modular manufacturing—a sector expected to grow as biopharma firms prioritize agility. The ability to replicate this model across regions also creates a recurring revenue stream, as partners like argenx seek localized production to meet regulatory and patient-centric demands.
Industry Implications and Risks
While the partnership is a win for both parties, investors should remain cognizantCTSH-- of risks. The biopharma industry is capital-intensive, and delays in Holly Springs' 2028 timeline could strain argenx's supply chain. Additionally, the modular manufacturing model's success hinges on regulatory acceptance of standardized processes across jurisdictions. However, given the kojoX network's alignment with the FDA's 2023 guidance on modular biomanufacturing, these hurdles appear manageable [5].
Conclusion
The FUJIFILM-argenx alliance exemplifies how strategic partnerships can harmonize innovation, scalability, and resilience. By anchoring VYVGART's production in a modular, localized network, the collaboration not only addresses current market demands but also future-proofs against industry volatility. For investors, the partnership's long-term value lies in its ability to capitalize on a $5.5 billion market, leverage therapeutic diversification, and pioneer a new paradigm in biopharma manufacturing. As the kojoX network expands, it may well become a blueprint for the next generation of supply chains—one that prioritizes proximity to patients without sacrificing global reach.

Comentarios
Aún no hay comentarios