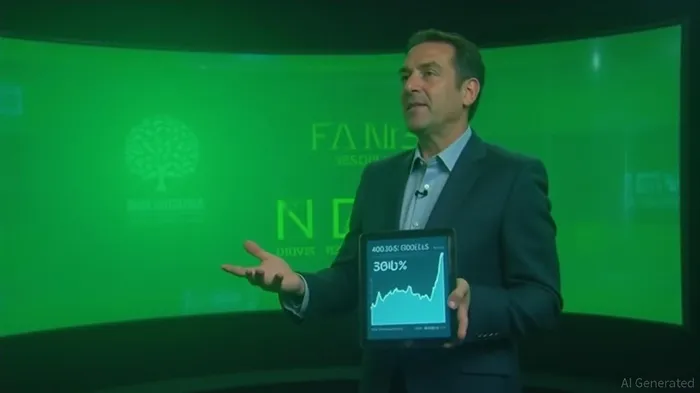
Spruce Biosciences, Inc. shares rise over 310.0%, currently trading at $35.94
PorAinvest
lunes, 6 de octubre de 2025, 9:37 am ET1 min de lectura
SPRB--
The FDA Breakthrough Therapy Designation is granted to therapies that address serious or life-threatening conditions and demonstrate substantial improvement over existing therapies. This designation provides Spruce Biosciences with more intensive FDA guidance, cross-disciplinary collaboration, and eligibility for rolling submission and priority review, potentially accelerating the path to market for TA-ERT .
Sanfilippo Syndrome Type B is an ultra-rare, fatal genetic disease affecting fewer than 1:200,000 people in the United States. The condition is characterized by deficiency in N-Acetyl-Alpha-Glycosaminidase, an enzyme required for breaking down heparan sulfate in lysosomes. Currently, there are no FDA-approved therapies for the condition, with management limited to palliative care. The FDA confirmed that CSF HS-NRE could serve as a surrogate biomarker reasonably likely to predict clinical benefit, potentially enabling accelerated approval .
Spruce Biosciences, a late-stage biopharmaceutical company focused on developing therapies for neurological disorders, remains on track to submit its Biologics License Application for TA-ERT in the first quarter of 2026. "This designation highlights TA-ERT’s potentially transformative clinical impact as the first disease-modifying therapy to treat MPS IIIB in children impacted by this devastating condition," said Javier Szwarcberg, Chief Executive Officer of Spruce Biosciences .
This news has significantly boosted investor confidence in Spruce Biosciences, leading to a substantial increase in its stock price. The company's focus on developing innovative treatments for rare and life-threatening conditions has positioned it as a leader in the biopharmaceutical sector.
Spruce Biosciences, Inc. shares rise over 310.0%, currently trading at $35.94
Spruce Biosciences, Inc. (NASDAQ: SPRB) experienced a significant surge in its stock price, rising over 310% and currently trading at $35.94. The dramatic increase is attributed to the company's announcement of receiving Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) for its tralesinidase alfa enzyme replacement therapy (TA-ERT) for Sanfilippo Syndrome Type B .The FDA Breakthrough Therapy Designation is granted to therapies that address serious or life-threatening conditions and demonstrate substantial improvement over existing therapies. This designation provides Spruce Biosciences with more intensive FDA guidance, cross-disciplinary collaboration, and eligibility for rolling submission and priority review, potentially accelerating the path to market for TA-ERT .
Sanfilippo Syndrome Type B is an ultra-rare, fatal genetic disease affecting fewer than 1:200,000 people in the United States. The condition is characterized by deficiency in N-Acetyl-Alpha-Glycosaminidase, an enzyme required for breaking down heparan sulfate in lysosomes. Currently, there are no FDA-approved therapies for the condition, with management limited to palliative care. The FDA confirmed that CSF HS-NRE could serve as a surrogate biomarker reasonably likely to predict clinical benefit, potentially enabling accelerated approval .
Spruce Biosciences, a late-stage biopharmaceutical company focused on developing therapies for neurological disorders, remains on track to submit its Biologics License Application for TA-ERT in the first quarter of 2026. "This designation highlights TA-ERT’s potentially transformative clinical impact as the first disease-modifying therapy to treat MPS IIIB in children impacted by this devastating condition," said Javier Szwarcberg, Chief Executive Officer of Spruce Biosciences .
This news has significantly boosted investor confidence in Spruce Biosciences, leading to a substantial increase in its stock price. The company's focus on developing innovative treatments for rare and life-threatening conditions has positioned it as a leader in the biopharmaceutical sector.
Divulgación editorial y transparencia de la IA: Ainvest News utiliza tecnología avanzada de Modelos de Lenguaje Largo (LLM) para sintetizar y analizar datos de mercado en tiempo real. Para garantizar los más altos estándares de integridad, cada artículo se somete a un riguroso proceso de verificación con participación humana.
Mientras la IA asiste en el procesamiento de datos y la redacción inicial, un miembro editorial profesional de Ainvest revisa, verifica y aprueba de forma independiente todo el contenido para garantizar su precisión y cumplimiento con los estándares editoriales de Ainvest Fintech Inc. Esta supervisión humana está diseñada para mitigar las alucinaciones de la IA y garantizar el contexto financiero.
Advertencia sobre inversiones: Este contenido se proporciona únicamente con fines informativos y no constituye asesoramiento profesional de inversión, legal o financiero. Los mercados conllevan riesgos inherentes. Se recomienda a los usuarios que realicen una investigación independiente o consulten a un asesor financiero certificado antes de tomar cualquier decisión. Ainvest Fintech Inc. se exime de toda responsabilidad por las acciones tomadas con base en esta información. ¿Encontró un error? Reportar un problema

Comentarios
Aún no hay comentarios