Soligenix's $7.5M Raise: A Strategic Move in a Fragmented Biotech Funding Landscape
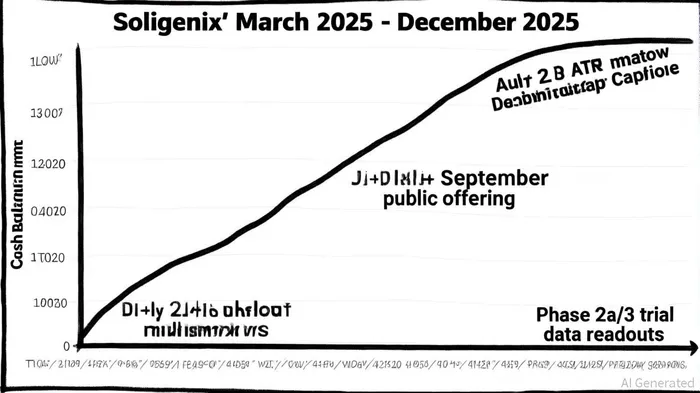
In September 2025, SoligenixSNGX--, Inc. (Nasdaq: SNGX) priced a $7.5 million public offering of common stock and warrants, raising capital to advance its pipeline of rare disease therapies. The offering, which includes 5,555,560 shares at $1.35 apiece and accompanying warrants exercisable at the same price, underscores the company's efforts to secure funding amid a biotech landscape increasingly dominated by megafunds and late-stage validation[2]. While the raise appears modest compared to the $93 million median deal size reported in 2025 Q3[5], Soligenix's strategy reflects a calculated alignment with industry trends favoring de-risked assets and milestone-driven capital allocation.
Industry Trends: The Rise of Megarounds and Therapeutic Platforms
The biotech sector in 2025 is characterized by a bifurcated capital market. On one side, venture capitalists are concentrating bets on late-stage programs with clear regulatory pathways, as evidenced by Sparrow Therapeutics' $95 million Series B for a Type 2 diabetes treatment[1] and NRG Therapeutics' £50 million raise for neurodegenerative disease candidates[1]. On the other, early-stage firms face tighter scrutiny, with Series A and B rounds shrinking in size compared to 2024[5]. Investors now prioritize therapeutic platforms—scalable technologies applicable across multiple disease areas—over single-asset companies[4]. This shift is driven by the desire to mitigate R&D risks and capitalize on long-term commercial potential.
Soligenix's Strategic Positioning
Soligenix's recent capital raise must be viewed through the lens of its pipeline and financial position. As of June 30, 2025, the company held $5.1 million in cash, down from $7.3 million in March 2025[3]. A July ATM facility added $1.4 million, but the September offering extends its runway through December 2025[3]. The proceeds will fund Phase 3 trials for HyBryte™ (synthetic hypericin) in cutaneous T-cell lymphoma (CTCL), with top-line results expected in late 2026[3], as well as Phase 2a studies in psoriasis and Behçet's Disease.
The offering's structure—combining shares and warrants—aligns with industry norms for mid-stage biotechs. By adjusting existing warrants to the $1.35 price point, Soligenix reduces dilution for new investors while maintaining flexibility for future financing[2]. However, the $7.5 million raise pales in comparison to the $100+ million megarounds dominating the sector[5]. This discrepancy highlights the challenges faced by smaller biotechs: while Soligenix's pipeline includes validated assets (e.g., HyBryte's Phase 3 CTCL trial), its lack of a broad therapeutic platform may limit its appeal to investors seeking scalable, multi-disease solutions[4].
Risk and Reward: Milestones as Catalysts
The success of Soligenix's capital raise hinges on its ability to deliver near-term data. Positive Phase 2a results for SGX302 (psoriasis) by year-end 2025[3] and the FLASH2 Phase 3 trial for HyBryte in CTCL (H1 2026) could catalyze partnerships or additional financing. Such milestones would align with broader industry dynamics, where clinical validation often triggers follow-on investments or big pharma interest[4]. Conversely, delays or negative data could strain the company's $7.5 million runway, particularly if the Phase 3 CTCL trial requires more resources than anticipated.
Conclusion: A Prudent but Narrow Path
Soligenix's $7.5 million offering is a pragmatic step in a capital-intensive sector. While it secures immediate funding for critical trials, the company's reliance on smaller raises contrasts with the megafund-driven strategies of peers like Sparrow or NRG Therapeutics[1]. For Soligenix, the key lies in leveraging its pipeline milestones to attract larger partnerships or follow-on capital. In a market increasingly defined by scale and platform potential, the company's success will depend on demonstrating that its rare disease focus—and the associated unmet medical needs—can translate into durable commercial value.

Comentarios
Aún no hay comentarios