Senti Bio's Biotech Showcase Panel: A Tactical Setup for a Data-Driven Move
The immediate catalyst is clear. SentiSNTI-- Bio's CEO, Dr. Timothy Lu, will take the stage at Biotech Showcase 2026 on Tuesday, January 13th. This isn't a routine presentation; it's a panel discussion titled "Engineering the Future: Advances in Cell and Gene Therapies," placing Senti's Gene Circuit platform directly in the spotlight of a premier investor event. The timing is strategic, as the conference runs during J.P. Morgan Healthcare Week, the industry's annual bellwether.
This setup offers a concentrated, high-visibility audience. The event is expected to draw more than 3,000 biotech leaders and 1,200+ investors. For a clinical-stage company like Senti, this is a prime opportunity to reinforce its narrative to a crowd primed for dealmaking. The panel's focus on cell and gene therapy advances provides perfect context for highlighting SENTI-202's promising clinical data and its regulatory momentum.
The tactical move here is about narrative reinforcement. By participating in this high-profile forum, Senti aims to solidify its position as a platform innovator in a crowded field. The goal is to translate that visibility into tangible investor interest or, more importantly, dealmaking momentum. Yet, the direct impact on valuation hinges on what happens next. A strong panel performance can spark conversations and meetings, but the stock's move will depend on whether those interactions lead to concrete follow-through, such as new partnerships or increased analyst coverage. For now, the event is a setup for a potential catalyst, not the catalyst itself.
The Asset: SENTI-202's Clinical and Regulatory Momentum
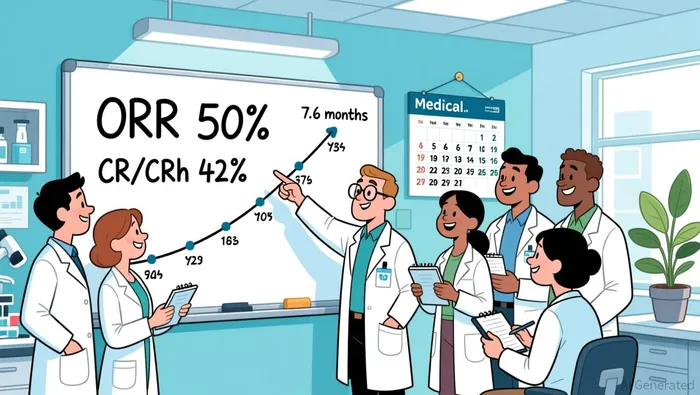
The panel discussion is a stage for a compelling data story. The fundamental driver is SENTI-202's clinical profile, which has already earned significant regulatory validation. The core metrics from the 20-patient cohort presented at ASH 2025 are striking: a 50% Overall Response Rate (ORR) and a 42% Complete Remission (CR)/CRh rate at the Recommended Phase 2 Dose. More importantly, the responses appear deep and durable, with a median duration of composite Complete Remission of 7.6 months. The safety profile is a key differentiator, with no dose-limiting toxicities and a manageable side-effect profile that supports the potential for outpatient delivery.
This clinical momentum has directly translated into regulatory milestones. The FDA's recent Regenerative Medicine Advanced Therapy (RMAT) designation is the second major recognition for SENTI-202 this year, following an Orphan Drug Designation granted in June. This dual designation is more than a formality; it signals the agency sees preliminary clinical evidence of potential to address unmet needs in relapsed/refractory AML. It also opens a pathway for accelerated development, with the FDA committed to close collaboration on generating pivotal data.
Viewed together, the data and the designations create a powerful narrative. The clinical results demonstrate a novel mechanism of action with selectivity for cancer cells, while the regulatory status provides a clear, fast-tracked path forward. For the Biotech Showcase panel, this is the tangible foundation to discuss. The event is a chance to reinforce this momentum to a captive audience of potential partners and investors. The setup is complete: strong data, regulatory validation, and a platform story. The next move will be about translating that narrative into dealmaking.
The Setup: Immediate Mechanics and Risk/Reward
The mechanics of this event are straightforward. Dr. Lu will present on a panel to more than 3,000 biotech leaders and 1,200+ investors during a week known for dealmaking. The goal is narrative reinforcement: to translate SENTI-202's clinical data and regulatory momentum into a clearer investment thesis for a concentrated audience. The risk is that it remains a "talking shop" with no immediate announcements of funding or partnerships.
For the stock, the move hinges on whether the panel successfully de-risks the investment thesis. The tactical setup is about perception. Strong data and regulatory designations are the foundation, but the event is the stage to discuss them with potential partners and investors who can influence the stock's trajectory. The immediate catalyst is the visibility and the potential for follow-on meetings that could spark conversations about collaboration or investment.
The key risk is that the event does not create a tangible catalyst. Without a new partnership, funding round, or significant analyst upgrade in the near term, the stock may see only a temporary pop on the event day before reverting. The panel provides a platform to reinforce the narrative, but the stock's reaction will depend on whether that narrative is perceived as de-risked enough to warrant a new investment thesis. The event is a setup, but the real test is what happens after the panel ends.
Catalysts and What to Watch
The event-driven thesis now hinges on specific, near-term outcomes. The panel is a setup; the catalysts are what follows. The key guardrail is clear: the absence of new clinical data or regulatory updates will limit the event's impact to mere narrative reinforcement. The stock's move will depend on whether the panel sparks tangible dealmaking conversations.
Watch for any mention of partnership discussions or funding milestones during or immediately after the panel. The Biotech Showcase platform is explicitly designed for unmatched opportunity to forge critical investment and partnering relationships. If Dr. Lu hints at or confirms active talks with potential partners, that would be a direct validation of the setup. Conversely, silence on collaboration would suggest the event failed to generate new interest.
Monitor the stock price reaction on January 13th and the following days for sustained momentum beyond a one-day pop. A successful event should create a window of heightened visibility and investor interest. A sustained rally would indicate the narrative was well-received and de-risked. A sharp reversal would signal that the market saw no new catalyst, treating the panel as a routine presentation.
The bottom line is that the panel provides a stage, but the stock needs a script. The immediate watch is on whether the script includes new partnership talks or funding commitments. Without those, the event's impact is likely to be fleeting.

Comentarios
Aún no hay comentarios