Securities Litigation Risks at Cytokinetics, Inc.: A Cautionary Tale for Investors
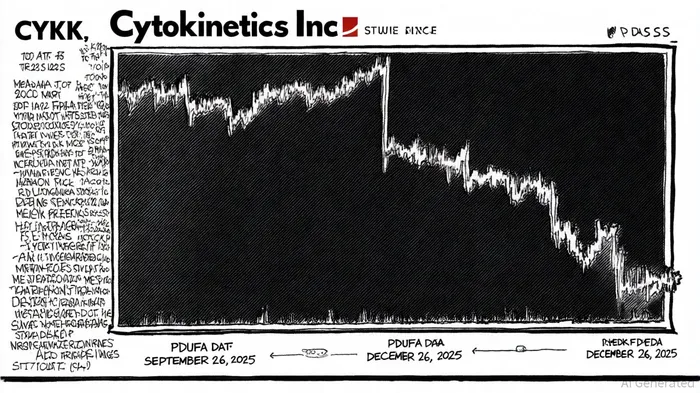
Investors evaluating CytokineticsCYTK--, Inc. (NASDAQ: CYTK) must navigate a complex web of regulatory and legal risks, particularly in light of recent securities litigation. A class action lawsuit filed against the company alleges material misrepresentations regarding its New Drug Application (NDA) for aficamten, a drug candidate for treating hypertrophic cardiomyopathy. According to a report by Robbins LLP, the lawsuit claims that Cytokinetics and its executives misled investors by failing to disclose risks tied to the omission of a Risk Evaluation and Mitigation Strategy (REMS) in the initial NDA submission, despite prior discussions with the FDA about its necessity [1]. This omission triggered an extended regulatory review period, pushing the Prescription Drug User Fee Act (PDUFA) target action date from September 26, 2025, to December 26, 2025 [2].
The implications for investor due diligence are profound. Data from Scott+Scott indicates that the company's stock price experienced a significant decline following the FDA's delay announcement in May 2025, eroding investor confidence and raising questions about corporate transparency [3]. The lawsuit, which covers purchases of CYTKCYTK-- stock between December 27, 2023, and May 6, 2025, underscores the importance of scrutinizing management's communication with regulators and the market [4]. For instance, Cytokinetics' February 2025 update—stating that the NDA was under FDA review with a PDUFA date of September 2025—omitted critical details about REMS-related risks, which later proved pivotal in delaying approval [5].
Regulatory scrutiny is not new for Cytokinetics. Between 2020 and 2025, the company faced multiple investigations and SEC filings that highlighted operational and compliance challenges. As stated by the Portnoy Law Firm, these historical issues, combined with the recent litigation, amplify the risks for shareholders who may face prolonged uncertainty and potential financial losses [6]. The current lawsuit also raises broader concerns about the company's risk management practices, particularly in high-stakes drug development timelines where REMS requirements are often non-negotiable.
For investors, the case serves as a stark reminder of the need for rigorous due diligence. Key considerations include:
1. Regulatory Alignment: Assess whether management's public statements align with known regulatory requirements. In CYTK's case, the failure to address REMS concerns proactively appears to have directly contributed to the FDA delay.
2. Transparency in Filings: Scrutinize SEC filings for gaps in risk disclosure. Cytokinetics' 10-K and 10-Q reports from 2024–2025, for example, did not adequately flag REMS-related challenges until after the fact .
3. Legal Exposure: Monitor the progress of the class action lawsuit, including the November 17, 2025, deadline for lead plaintiff motions. Legal outcomes could impact not only stock valuation but also the company's ability to secure future financing.
While Cytokinetics maintains that its NDA for aficamten remains under active FDA review, the litigation and regulatory setbacks highlight the fragility of its business model. For investors, the lesson is clear: in industries where regulatory outcomes are binary and time-sensitive, even minor oversights in communication can have cascading financial and legal consequences.

Comentarios
Aún no hay comentarios