Santhera's AGAMREE Approval in Canada: A Catalyst for Long-Term Growth in Rare Disease Therapeutics
Santhera Pharmaceuticals' AGAMREE (vamorolone) has achieved a landmark regulatory milestone in Canada, securing Health Canada's approval on October 3, 2025, as the first and only therapy for Duchenne muscular dystrophy (DMD) in patients aged 4 years and older[1]. This approval, granted under a Priority Review process, underscores the urgent unmet need in DMD treatment and positions AGAMREE as a transformative option for a rare disease affecting over 800 boys and young men in Canada[2]. For investors, the approval raises critical questions about Santhera's long-term commercial potential and its ability to scale AGAMREE's market penetration in a high-growth, niche therapeutic area.
Regulatory Momentum and Clinical Differentiation
AGAMREE's approval in Canada follows its U.S. and EU authorizations in 2023 and 2024, respectively[3], reflecting a consistent regulatory trajectory. The drug's novel mechanism-modifying glucocorticoid receptor activity to dissociate anti-inflammatory efficacy from steroid-related side effects-addresses key limitations of traditional corticosteroids, which are the current standard of care for DMD[4]. Clinical data from the pivotal Phase 2b VISION-DMD study demonstrated AGAMREE's ability to preserve bone health and growth while maintaining functional benefits, a critical differentiator in a patient population where long-term steroid use often leads to severe comorbidities[5].
This clinical edge, combined with Health Canada's Priority Review designation, has accelerated AGAMREE's market entry. Kye Pharmaceuticals, which holds exclusive Canadian commercial rights, has emphasized its commitment to ensuring broad patient access[6]. For Santhera, the Canadian approval reinforces its position as a leader in DMD innovation and validates the scalability of its commercial model.
Market Potential and Competitive Landscape
The Canadian DMD market, though small, is highly lucrative due to the high per-patient costs of orphan drug therapies. Catalyst Pharmaceuticals, which licenses AGAMREE for North America, has already demonstrated robust revenue growth: net product revenue in Q1 2025 reached $22.0 million, a 213% year-over-year increase[7]. Catalyst projects AGAMREE to generate $100–110 million in Canadian net sales for 2025, contributing significantly to its full-year revenue guidance of $545–565 million[8].
AGAMREE's first-mover advantage in Canada is a strategic win, as the country previously lacked an approved DMD therapy. While global competitors like Italfarmaco (Givinostat) and FibroGen (Pamrevlumab) are advancing their pipelines, AGAMREE's established safety profile and regulatory approvals provide a durable moat[9]. Moreover, Santhera's ongoing trials in younger DMD patients (aged 2–4 years) could further expand its addressable market[10].
Pipeline Depth and Rare Disease Synergies
Beyond AGAMREE, Santhera's pipeline highlights its commitment to rare diseases. The company is developing POL6014, a neutrophil elastase inhibitor for cystic fibrosis, and Pimasertib, an MEK1/2 inhibitor for ovarian cancer, both of which are in Phase 2 trials[11]. These programs, coupled with AGAMREE's potential expansion into other neuromuscular indications, create a diversified revenue stream.
Notably, Santhera's expertise in dissociative steroids and rare disease mechanisms positions it to leverage cross-therapeutic insights. For instance, AGAMREE's favorable bone health profile could inform its use in other steroid-dependent conditions, such as inflammatory bowel disease or asthma, broadening its commercial footprint[12].
Risks and Mitigants
Despite its strengths, Santhera faces challenges. The high cost of orphan drug development and reimbursement hurdles in public healthcare systems could constrain AGAMREE's uptake. However, Kye's experience in navigating Canadian reimbursement frameworks-evidenced by its successful commercialization of FIRDAPSE-mitigates this risk[13]. Additionally, Santhera's collaboration with Catalyst and Kye ensures shared financial and operational burdens, enhancing the likelihood of sustained growth.
Conclusion: A Compelling Long-Term Investment
Santhera's AGAMREE approval in Canada is more than a regulatory win-it is a testament to the company's ability to innovate in a high-margin, underserved market. With a projected $100–110 million revenue contribution in 2025 and a pipeline of rare disease candidates, Santhera is well-positioned to capitalize on the growing demand for precision therapies. For investors, the key takeaway is clear: Santhera's focus on mechanistically differentiated treatments and strategic partnerships creates a robust foundation for long-term value creation in the rare disease space. 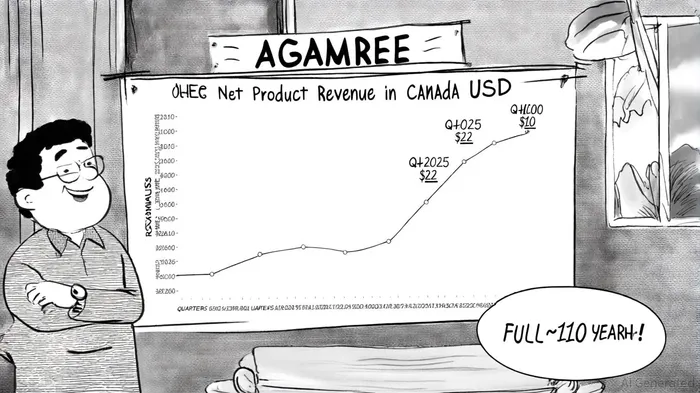



Comentarios
Aún no hay comentarios