Roivant and Priovant's Brepocitinib: A High-Conviction Biotech Play in Rare Autoimmune Diseases
In the high-stakes world of biotech investing, few opportunities combine near-term regulatory milestones with transformative market differentiation as compellingly as Roivant SciencesROIV-- and its subsidiary Priovant Therapeutics. Their lead asset, brepocitinib, a dual selective inhibitor of TYK2 and JAK1, is poised to redefine treatment paradigms for rare autoimmune conditions like dermatomyositis (DM) and non-infectious uveitis (NIU). With Phase 3 success in DM and a robust partnership ecosystem, this pipeline represents a rare convergence of clinical innovation, unmet medical need, and clear commercial pathways.
Brepocitinib in Dermatomyositis: A Clinical and Commercial Breakthrough
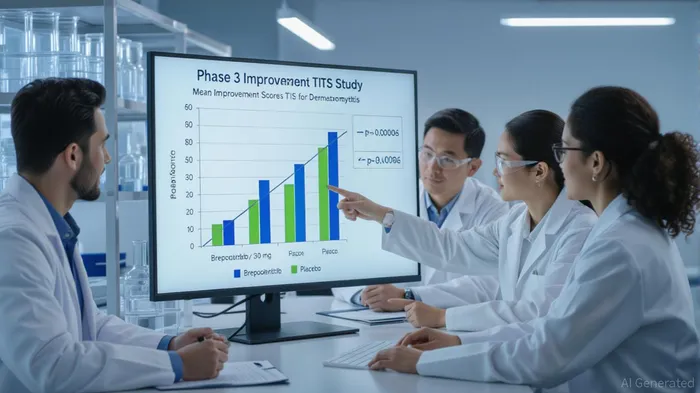
Dermatomyositis, a rare inflammatory disease affecting both skin and muscle, has long lacked effective therapies. Current treatments, including corticosteroids and broad-spectrum immunosuppressants, often fall short in addressing disease-specific pathology while causing significant side effects. Roivant and Priovant's Phase 3 VALOR study—the largest interventional trial in DM to date—has delivered a landmark result: brepocitinib 30 mg achieved a statistically significant improvement in the Total Improvement Score (TIS) at week 52, with a mean of 46.5 versus 31.2 for placebo (p=0.0006) [1]. Over two-thirds of patients on the high dose experienced at least a moderate response (TIS≥40), and nearly half achieved a major response (TIS≥60) [2].
The drug's steroid-sparing effect further strengthens its value proposition. By week 52, 42% of patients on brepocitinib 30 mg were able to discontinue steroids entirely, compared to 23% on placebo [3]. This addresses a critical unmet need, as prolonged steroid use exacerbates comorbidities like diabetes and osteoporosis. The consistent safety profile across trials—no new safety signals observed—adds to its appeal [4].
With a New Drug Application (NDA) filing slated for the first half of 2026, brepocitinib is on track to become the first approved oral therapy for DM in over a decade. Given the disease's prevalence of ~10–20 cases per million people and the absence of FDA-approved targeted therapies, Roivant and Priovant are positioned to capture a dominant market share.
Market Differentiation: Mechanism, Convenience, and Clinical Evidence
Brepocitinib's dual inhibition of TYK2 and JAK1 offers a mechanistic edge over monotherapies. By suppressing cytokines like IFN-α/β, IL-12, and IL-23, it directly targets the inflammatory pathways driving DM [5]. This specificity contrasts with existing JAK inhibitors, which often lack selectivity and carry higher toxicity risks.
Oral, once-daily dosing further differentiates brepocitinib from intravenous or injectable alternatives, improving patient adherence and reducing healthcare system burden. The molecule's rapid onset of action—evidenced by early improvements in skin and muscle scores—also aligns with patient and physician priorities [6].
Near-Term Catalysts and Strategic Partnerships
The NDA filing for DM in H1 2026 is the most immediate catalyst, with potential approval by late 2026 or early 2027. Parallel progress in non-infectious uveitis adds depth to the pipeline. The Phase 2 NEPTUNE study demonstrated brepocitinib's ability to reduce uveitic macular edema, a leading cause of vision loss in NIUNIU-- [7]. A Phase 3 CLARITY trial is set to begin in late 2024, with data potentially supporting another NDA filing by 2027.
Strategic partnerships amplify the commercial potential. Pfizer, which holds global development rights and U.S./Japan commercial rights to brepocitinib, also owns a 25% equity stake in Priovant [8]. This alignment of interests ensures robust financial backing and leverages Pfizer's commercial infrastructure for rapid market penetration.
Conclusion: A High-Conviction Play in a Fragmented Market
Roivant and Priovant's brepocitinib pipeline exemplifies the ideal biotech investment: a differentiated mechanism, clear regulatory milestones, and a focus on rare diseases with limited competition. The drug's dual indication potential in DM and NIU, coupled with Pfizer's partnership, creates a multi-billion-dollar commercial opportunity. For investors seeking exposure to near-term catalysts and transformative therapies, this is a rare and compelling opportunity.

Comentarios
Aún no hay comentarios