Roche's Breakthrough in Breast Cancer Therapies: Assessing Long-Term Market Potential and Competitive Edge
Roche's recent advancements in targeted therapies for breast cancer have positioned the company as a formidable player in the oncology space. With two pivotal phase III trials—evERA for giredestrant and INAVO120 for inavolisib (Itovebi)—yielding statistically significant improvements in progression-free survival (PFS) and overall survival (OS), Roche is not only addressing unmet medical needs but also reshaping the competitive landscape for ER-positive/HER2-negative and PIK3CA-mutated subtypes. This analysis evaluates the long-term market potential and strategic advantages of these therapies, supported by clinical data and market dynamics.
Clinical Breakthroughs: Precision and Efficacy
Roche's giredestrant, an oral selective estrogen receptor degrader (SERD), demonstrated statistically significant and clinically meaningful improvements in PFS in the evERA trial for patients with ER-positive, HER2-negative metastatic breast cancer previously treated with CDK 4/6 inhibitors and endocrine therapy. The all-oral combination with everolimus was well tolerated, with no new safety signals reported [1]. This positions giredestrant as a potential first-line option for patients with ESR1 mutations, a subgroup with historically poor outcomes.
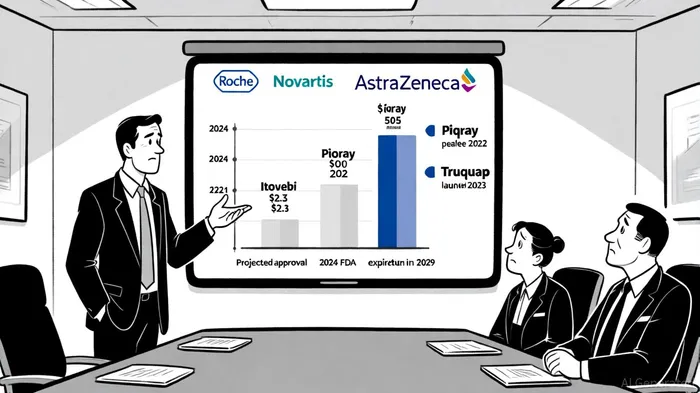
For Itovebi (inavolisib), the INAVO120 trial showed a 34-month median OS in PIK3CA-mutated, HR-positive, HER2-negative advanced breast cancer patients treated with the triplet regimen of inavolisib, palbociclib, and fulvestrant, compared to 27 months in the control group. The regimen also achieved a 33% reduction in the risk of death and a 17.2-month PFS versus 7.3 months [3]. These results, coupled with a favorable safety profile (e.g., 5.6% hyperglycemia vs. 36.6% with Piqray), underscore Itovebi's potential to displace existing PI3K inhibitors like Novartis' Piqray and AstraZeneca's Truqap [2].
Market Potential: Niche but High-Growth Segments
The HER-2 negative breast cancer market is projected to grow at a 9.17% CAGR, reaching $25.44 billion by 2030, driven by precision diagnostics, ADCs, and targeted therapies [1]. Within this, the PIK3CA-mutated subtype—present in 34–40% of HR+ breast cancers—represents a high-unmet-need segment. Roche's Itovebi, with its mutant-selective PI3Kα inhibition, is uniquely positioned to capture this niche. Analysts project Itovebi to achieve peak sales of $2.3 billion, leveraging its superior efficacy and tolerability over competitors [2].
The broader metastatic HR+/HER2- market, valued at $10 billion in 2023, is expected to expand further with the introduction of therapies like vepdegestrant (a PROTAC ER degrader) and next-gen PI3K inhibitors [3]. Roche's dual focus on giredestrant and Itovebi aligns with this trend, offering complementary mechanisms to address resistance pathways in advanced disease.
Competitive Positioning: Disrupting the Status Quo
Roche's Itovebi faces competition from Piqray (Novartis) and Truqap (AstraZeneca), but its clinical advantages are clear. Piqray's sales declined by 7% in H1 2024 to $229 million, partly due to Truqap's 2023 launch and Itovebi's 2024 FDA approval [2]. Itovebi's 57% reduction in progression/death risk in the INAVO120 trial, compared to Piqray's 36% in SOLAR-1, highlights its efficacy edge [2]. Additionally, Itovebi's lower toxicity profile (e.g., hyperglycemia) could drive adoption in community settings, where cost-effectiveness is critical.
Roche's pipeline depth further strengthens its position. With giredestrant's phase III readouts expected in 2025, the company is poised to dominate both ESR1-mutated and PIK3CA-mutated subtypes. Meanwhile, competitors like NovartisNVS-- face patent expirations (Piqray by 2029), creating a window for Roche to solidify market share [2].
Conclusion: A Strategic Win for Long-Term Investors
Roche's dual breakthroughs in giredestrant and Itovebi reflect a strategic pivot toward personalized, mechanism-specific therapies in breast cancer. With a $2.3 billion peak sales projection for Itovebi, favorable clinical data, and a growing market for targeted agents, Roche is well-positioned to outperform competitors in the high-growth HER-2 negative and PIK3CA-mutated segments. For investors, the combination of clinical differentiation, market expansion, and pipeline momentum makes Roche's oncology portfolio a compelling long-term bet.



Comentarios
Aún no hay comentarios