Ribomic's Phase 2 Cohort 2 Success in Achondroplasia: A Catalyst for Rare Disease Innovation
The recent announcement of positive results from Ribomic's Phase 2 Cohort 2 trial for umedaptanib pegol marks a pivotal moment in the development of orphan drugs for achondroplasia. This anti-FGF2 aptamer therapy demonstrated a mean height growth rate of +1.4 cm/year in pediatric patients, comparable to the 1.7 cm/year observed with BioMarin's Voxzogo, the current standard of care, according to a Coherent Market Insights report. However, the trial's standout outcomes were the two patients achieving +5.0 cm/year and +2.0 cm/year, respectively-figures that suggest the potential for a more potent therapeutic effect in a subset of patients. With no safety concerns reported and plans to advance to Phase 3 trials in early 2026, Ribomic has positioned itself as a formidable contender in a rapidly expanding market.
A High-Stakes Market for Rare Disease Innovation
The global achondroplasia treatment market is poised for explosive growth, projected to surge from USD 238.5 million in 2025 to USD 2,105.8 million by 2032, driven by a 36.5% compound annual growth rate (CAGR). This expansion is fueled by rising awareness of congenital diseases, advancements in gene-targeted therapies, and the approval of novel treatments like vosoritide and TransCon CNP. Japan, a key market for Ribomic, faces unique challenges, including delays in orphan drug approvals-a phenomenon known as "drug lag"-but also offers robust incentives such as market exclusivity and tax benefits to offset R&D costs, as described in a PubMed review. The country's orphan drug designation framework, aligned with global standards, provides a critical advantage for companies like Ribomic aiming to secure regulatory approval.
Navigating Regulatory and Competitive Landscapes
Japan's regulatory environment is evolving to address historical bottlenecks. The Pharmaceuticals and Medical Devices Agency (PMDA) and the Ministry of Health, Labour and Welfare (MHLW) have intensified collaborations with the European Medicines Agency (EMA) to streamline orphan drug designations and approvals, according to PMDA guidance. These efforts align with broader reforms to reduce drug lag, which has historically hindered access to innovative therapies in Japan. For Ribomic, the orphan drug designation in Japan not only accelerates regulatory pathways but also grants a competitive edge against global players like BioMarinBMRN-- and Ascendis Pharma A/S, which dominate the current treatment landscape.
Investment Implications: Balancing Risk and Reward
The clinical and regulatory milestones achieved by Ribomic present a compelling case for investors. The Phase 2 results, particularly the high-dose cohort's performance, suggest the potential to outperform existing therapies in specific patient populations. With plans to expand the Phase 3 trial to younger patients (around two years old) and increase dosing frequency, Ribomic is addressing unmet needs in early intervention-a critical factor in achondroplasia management. However, risks remain, including the high costs of orphan drug development and the need to demonstrate consistent efficacy across larger trials.
The market's projected growth, coupled with Japan's regulatory tailwinds, positions Ribomic to capture a significant share of the USD 2.1 billion market by 2032. Investors should also consider the company's strategic alignment with global trends, such as the shift toward patient-centric care models and the integration of digital tools for real-time data analysis, as highlighted in a PR Times briefing. These factors, combined with the orphan drug incentives in Japan, create a favorable environment for long-term value creation.
Conclusion
Ribomic's Phase 2 Cohort 2 success underscores the transformative potential of targeted therapies in rare diseases. While the path to commercialization involves navigating complex regulatory and competitive dynamics, the company's clinical progress and Japan's supportive ecosystem offer a strong foundation for growth. For investors, the key will be monitoring the Phase 3 trial outcomes and the broader adoption of orphan drug policies in Japan-a market that is increasingly central to the global rare disease innovation landscape.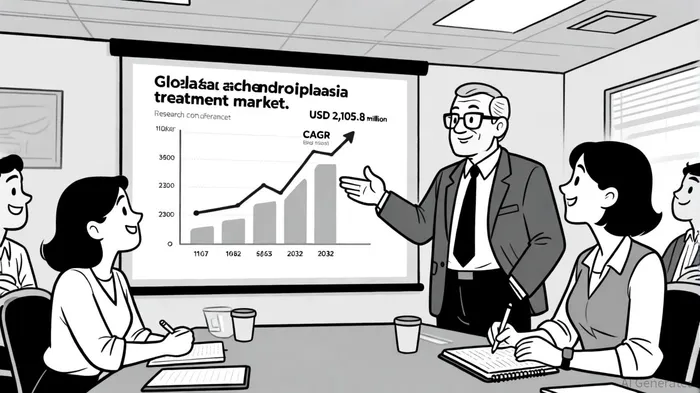

Comentarios
Aún no hay comentarios