Reviva Pharmaceuticals' Secondary Offering: Assessing IPO Readiness and Long-Term Growth in a Dynamic Biopharma Sector
Reviva Pharmaceuticals Holdings, Inc. (NASDAQ: RVPH) has recently announced a public offering of common stock and warrants, signaling its strategic intent to bolster capital reserves for research and development (R&D) and general corporate purposes[1]. While the company is already publicly traded, this secondary offering—managed by A.G.P./Alliance Global Partners—reflects its broader ambition to scale operations ahead of a potential New Drug Application (NDA) submission for its lead candidate, brilaroxazine, in Q2 2026[1]. This article evaluates Reviva's readiness for further capital-raising efforts, its financial health, and its positioning within the evolving schizophrenia drug market.
Clinical Progress and Regulatory Pathway
Reviva's schizophrenia drug, brilaroxazine (RP5063), has demonstrated robust clinical outcomes in Phase 3 trials. The RECOVER open-label extension (OLE) study, which enrolled 446 patients, reported a -18.1-point reduction in the PANSS total score over one year, alongside significant improvements in negative symptoms and neuroinflammatory biomarkers[2]. These results, coupled with a favorable safety profile (35% discontinuation rate after one year), position brilaroxazine as a potential best-in-class therapy[2]. The company plans an End-of-Phase 3 meeting with the FDA in Q4 2025 to discuss the regulatory pathway, with the goal of avoiding an additional Phase 3 trial[3].
The schizophrenia drug market is highly competitive, with emerging therapies such as Minerva Neurosciences' roluperidone and Karuna Therapeutics' KarXT (acquired by Bristol Myers Squibb) vying for market share[4]. However, brilaroxazine's unique mechanism as a serotonin-dopamine signaling modulator, combined with its broad receptor activity and favorable drug-drug interaction profile, offers differentiation[4].
Financial Health and Capital Needs
Reviva's financials reveal a mixed picture. For Q2 2025, the company reported a net loss of $6.1 million, or $0.12 per share, compared to $7.9 million in the same period in 2024[5]. Cash reserves stood at $10.4 million as of June 30, 2025, down from $13.5 million in late 2024[5]. To address liquidity needs, RevivaRVPH-- raised $10.0 million in Q2 2025 through a public equity offering[5]. The recent secondary offering, priced at $0.50 per share in June 2025, aims to raise an additional $10 million, underscoring the company's reliance on dilutive financing to fund operations[6].
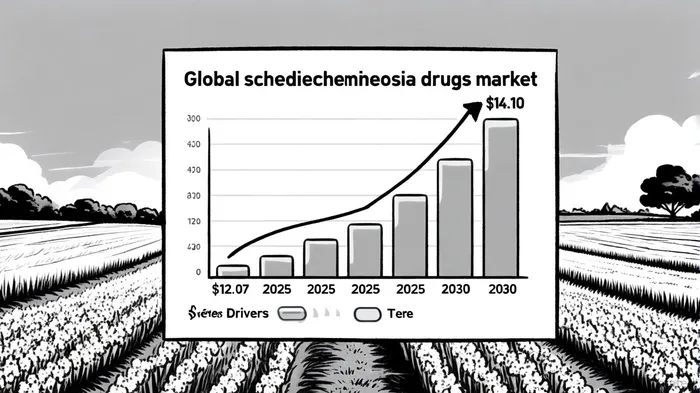
Market Dynamics and Long-Term Potential
The global schizophrenia drugs market is projected to grow at a compound annual growth rate (CAGR) of 3.15% from 2025 to 2030, reaching $14.10 billion by 2030[7]. This growth is driven by rising prevalence, advancements in long-acting injectable (LAI) formulations, and improved mental health insurance861218-- coverage[7]. Second-generation antipsychotics currently dominate the market, but third-generation therapies—like brilaroxazine—are gaining traction due to reduced side effects and enhanced efficacy[8].
Reviva's focus on biomarker-driven development and vocal profile analyses further strengthens its competitive edge[9]. However, the company must navigate challenges such as regulatory uncertainty, intense competition, and the high cost of late-stage clinical trials. A successful NDA submission in 2026 would be critical to unlocking commercial value, though approval timelines and post-marketing requirements remain key risks.
IPO Readiness and Strategic Considerations
While Reviva is not pursuing a traditional IPO, its secondary offering and ongoing R&D efforts suggest a strategy to build investor confidence ahead of potential future capital raises. The company's shelf registration on Form S-3 provides flexibility to access capital markets efficiently[1]. However, its reliance on equity financing raises concerns about shareholder dilution and long-term profitability.
For investors, Reviva's prospects hinge on three factors: (1) the FDA's receptiveness to its NDA, (2) the ability to differentiate brilaroxazine in a crowded market, and (3) the sustainability of its financial model. If approved, brilaroxazine could capture a meaningful share of the schizophrenia market, particularly given the unmet need for therapies addressing negative and cognitive symptoms[10].
Conclusion
Reviva Pharmaceuticals is navigating a pivotal phase in its development, with clinical and regulatory milestones poised to define its trajectory. While its financial position remains precarious, the company's robust clinical data and strategic capital-raising efforts position it to capitalize on the growing schizophrenia drug market. Investors should closely monitor the FDA meeting in Q4 2025 and the subsequent NDA submission, which will serve as critical inflection points for the stock. In a biopharma landscape marked by innovation and competition, Reviva's success will depend on its ability to execute its regulatory strategy and demonstrate the commercial viability of brilaroxazine.

Comentarios
Aún no hay comentarios