Replimune's (REPL) Turbulent Path: Assessing Long-Term Risks and Strategic Implications in Biotech's High-Stakes Environment
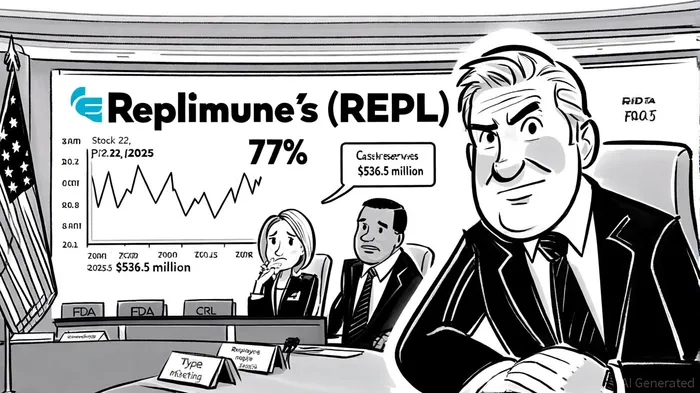
The biotechnology sector has long been a theater of high-stakes gambles, where the success or failure of a single clinical candidate can redefine a company's trajectory. Replimune GroupREPL--, Inc. (NASDAQ: REPL) is now at a pivotal juncture following the U.S. Food and Drug Administration's (FDA) rejection of its Biologics License Application (BLA) for RP1, a flagship oncolytic immunotherapy for advanced melanoma. This regulatory setback, coupled with a securities class-action lawsuit and a 77% stock price collapse, raises critical questions about the company's long-term viability and the broader risks inherent in biotech's innovation-driven model.
Regulatory Setbacks and Trial Design Flaws
The FDA's Complete Response Letter (CRL), issued on July 22, 2025, marked a decisive turning point. According to a report by Bloomberg, the agency concluded that Replimune's IGNYTE trial—a pivotal study supporting the BLA—was “not considered to be an adequate and well-controlled clinical investigation” due to patient heterogeneity and an inability to isolate RP1's efficacy in combination therapy [1]. This critique underscores a growing regulatory emphasis on rigorous trial design, particularly under the leadership of the FDA's Center for Biologics Evaluation and Research (CBER), which has adopted a more stringent posture in recent months [2].
Replimune's subsequent Type A meeting with the FDA in September 2025 yielded no clear path forward for accelerated approval [3]. While the company maintains that RP1's risk-benefit profile remains compelling, the absence of regulatory alignment highlights the precariousness of relying on single-product pipelines in a sector where trial design flaws can derail years of progress.
Legal Liabilities and Shareholder Value Erosion
The CRL's public disclosure triggered not only a market rout but also a securities class-action lawsuit. As stated by Hagens Berman, the lawsuit alleges that ReplimuneREPL-- and its executives misled investors by overstating the IGNYTE trial's prospects, despite internal awareness of its methodological shortcomings [4]. The class period spans from November 22, 2024, to July 21, 2025, with a lead plaintiff deadline of September 22, 2025 [5]. While no definitive liability estimates have been disclosed, the litigation's outcome could impose significant financial and reputational costs, particularly if the court rules that the company engaged in material misrepresentation.
The stock's collapse—a 77% drop on July 22, 2025—reflects the market's loss of confidence. Even with $536.5 million in cash reserves as of December 2024, Replimune's ability to fund its pipeline and navigate legal expenses without diluting shareholder value remains uncertain [6]. This scenario illustrates a broader risk in biotech: the compounding effects of regulatory and legal headwinds on capital structure and investor sentiment.
Strategic Implications and Pipeline Resilience
Replimune's financial position, however, is not without resilience. The company has initiated trials for RP2 in metastatic uveal melanoma and hepatocellular carcinoma, and its cash reserves suggest it can sustain operations for at least 18 months [7]. Yet, the absence of a clear regulatory pathway for RP1—a drug that had been positioned as a commercial cornerstone—casts doubt on the pipeline's ability to deliver near-term returns.
The case of Replimune also highlights the strategic importance of diversification. While the company's oncolytic platform shows promise, its overreliance on RP1 mirrors the vulnerabilities seen in other biotech firms that have faced similar setbacks. As noted by industry analysts, firms with multi-product pipelines and robust preclinical assets are better positioned to weather regulatory storms [8].
Broader Lessons for Biotech Investors
Replimune's turmoil serves as a cautionary tale for investors. First, it underscores the necessity of scrutinizing trial design and regulatory alignment, particularly for companies pursuing accelerated approval pathways. Second, it highlights the legal risks associated with aggressive clinical messaging, which can backfire if trial outcomes fall short of expectations. Finally, it reinforces the importance of financial prudence: even well-capitalized firms can face existential threats when regulatory and legal challenges converge.
For Replimune, the path forward hinges on its ability to restructure its pipeline, secure regulatory clarity for RP1, and mitigate legal exposure. However, the company's experience reflects a broader reality in biotech: innovation is inherently risky, and the line between breakthrough and failure is often razor-thin. Investors must weigh these risks carefully, recognizing that while the sector offers transformative potential, it demands a disciplined, long-term perspective.

Comentarios
Aún no hay comentarios