RAP-219's Phase 2a Readout and Its Strategic Implications for Rapport Therapeutics’ Precision Neuroscience Platform
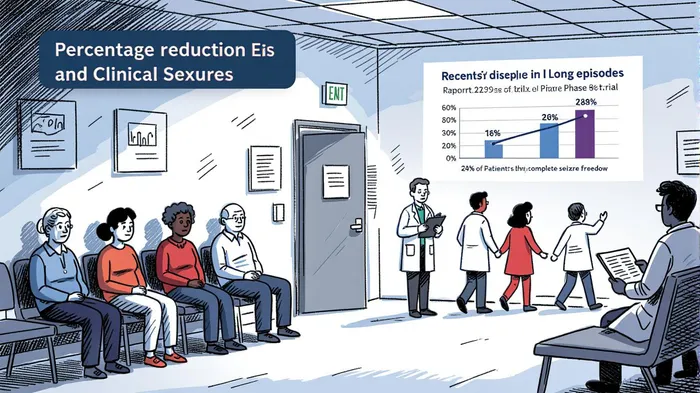
The biotech sector is no stranger to volatility, but when a clinical-stage company like RapportRAPP-- Therapeutics delivers a Phase 2a readout that defies expectations, it’s time to sit up and take notice. Rapport’s RAP-219, a TARPγ8-specific AMPAR negative allosteric modulator, just did exactly that. According to a report by Financial Content, the drug demonstrated statistically significant reductions in both long episodes (LEs) and clinical seizures in patients with drug-resistant focal onset seizures, with 85.2% of patients achieving a ≥30% reduction in LEs (p<0.0001) and 24% achieving complete seizure freedom over 8 weeks [1]. These results aren’t just impressive—they’re transformative, especially for a market where existing therapies often fall short.
A Precision Play in a Broad-Acting World
Rapport’s precision neuroscience platform is built on a simple yet revolutionary premise: target specific receptor-associated proteins (RAPs) to modulate neural circuits with surgical precision. Unlike traditional anti-seizure medications, which broadly suppress brain activity and risk systemic side effects, RAP-219’s mechanism allows it to act selectively on TARPγ8-expressing regions of the brain, minimizing off-target effects [3]. This isn’t just incremental innovation—it’s a paradigm shift. Data from the Phase 2a trial underscore this, showing that RAP-219 was well tolerated, with most adverse events mild or moderate and no serious side effects reported [1]. For investors, this means a lower regulatory risk profile and a clearer path to commercialization.
Catalyst Potential: From Epilepsy to a CNS Empire
The epilepsy market is a $10 billion global opportunity, but Rapport’s ambitions—and its platform’s potential—extend far beyond. The company is already advancing RAP-219 into Phase 2 trials for bipolar disorder, a condition where current treatments often lack efficacy or carry significant psychiatric risks [4]. This diversification isn’t speculative; it’s strategic. By leveraging the same mechanism that worked in epilepsy, Rapport is testing whether TARPγ8 modulation can stabilize mood pathways in bipolar patients. If successful, this would validate the platform’s versatility and open doors to a $15 billion bipolar market.
Moreover, Rapport’s cash reserves of $260.4 million as of Q2 2025 provide a financial runway through 2026, ensuring it can fund Phase 3 trials for epilepsy and expand into new indications without immediate dilution [4]. This stability is critical in biotech, where cash burn often derails even the most promising pipelines.
Strategic Alliances and Scientific Heft
Rapport’s partnerships with Third Rock Ventures and Johnson & Johnson Innovation aren’t just about funding—they signal industry validation of the platform’s potential [3]. But the real proof lies in the science. The company’s advisory board includes Nobel laureates and neuroscience heavyweights, a testament to the rigor underpinning its approach. As one analyst noted, “Rapport isn’t just chasing a single drug; they’re building a toolkit for precision neuromedicine” [3].
Risks and Realities
No stock is without risks. The FDA recently placed a clinical hold on RAP-219’s trial for diabetic peripheral neuropathic pain, citing protocol concerns [2]. While this is a setback, it’s a minor detour compared to the epilepsy and bipolar programs. Investors should also monitor Phase 3 trial design for RAP-219 in epilepsy, expected to begin in Q3 2026. A well-structured trial with clear endpoints will be critical to maintaining momentum.
The Bottom Line: A Catalyst-Driven Story
For long-term investors, Rapport Therapeutics offers a rare combination: a validated platform, a near-term catalyst (Phase 3 trials), and a diversified pipeline. The Phase 2a results in epilepsy are a green light for expansion, and the company’s scientific and financial foundations are robust. With a market cap that still reflects early-stage risk, RAPP is positioned to reap outsized rewards if RAP-219 secures approval.
In a sector where hope often outpaces reality, Rapport’s precision play is a rare blend of innovation and execution. For those willing to ride the wave, the next 12–18 months could be transformative.
Source:
[1] Rapport Announces Positive Topline Results from Phase 2a Clinical Trial of RAP-219 in Patients with Focal Onset Seizures [https://markets.financialcontent.com/wral/article/gnwcq-2025-9-8-rapport-announces-positive-topline-results-from-phase-2a-clinical-trial-of-rap-219-in-patients-with-focal-onset-seizures]
[2] FDA Halts Rapport Therapeutics' RAP-219 Trial for Diabetic Peripheral Neuropathic Pain [https://trial.medpath.com/news/ee3be96a50578ae1/fda-places-clinical-hold-on-epilepsy-agent-rap-219-for-diabetic-peripheral-neuropathic-pain]
[3] What are the primary areas of focus for Rapport Therapeutics? [https://synapse.patsnap.com/article/what-are-the-primary-areas-of-focus-for-rapport-therapeutics]
[4] Rapport Therapeutics Reports Second Quarter 2025 Financials and Provides Business Update [https://www.stocktitan.net/news/RAPP/rapport-therapeutics-reports-second-quarter-2025-financials-and-3wf9w5qi8j45.html]

Comentarios
Aún no hay comentarios