Quince Therapeutics: Strategic Differentiation and Investor Sentiment in the mRNA Therapeutics Race
Quince Therapeutics (QNCX) has emerged as a compelling case study in strategic differentiation within the mRNA therapeutics space, leveraging its focus on rare diseases and proprietary platform technologies to carve out a niche in a rapidly expanding market. The company's recent H.C. Wainwright 27th Annual Global Investment Conference presentation in September 2025 underscored its progress in advancing its lead asset, encapsulated dexamethasone sodium phosphate (eDSP), for the treatment of Ataxia-Telangiectasia (A-T), a rare pediatric neurodegenerative disorder[1].
Market Differentiation: Precision and Partnerships
Quince's differentiation strategy hinges on two pillars: proprietary bioinformatics tools and strategic partnerships. The company has developed advanced algorithms to enhance neoantigen hit rates, a critical factor in personalized cancer immunotherapy[3]. This approach addresses a key challenge in mRNA-based therapies—ensuring the immune system recognizes and targets specific disease markers. Additionally, Quince's superior lipid formulations for dendritic-cell targeting improve the efficacy of its mRNA payloads, setting it apart from competitors who rely on generic delivery systems[3].
The partnership with Option CareOPCH-- Health further solidifies Quince's market position. By securing a nationwide specialty infusion network for eDSP, the company ensures scalable access to patients post-approval, a critical factor in commercial success for rare disease therapies[1]. This infrastructure advantage is rare among small-cap biotechs and positions QuinceQNCX-- to capture a significant share of the A-T treatment market, which is currently underserved.
Investor Sentiment: A Post-H.C. Wainwright Analysis
While direct metrics like stock price movements or analyst ratings post-presentation are not publicly available, Quince's strategic disclosures suggest a calculated effort to bolster investor confidence. The completion of its pivotal Phase 3 NEAT trial—enrolling 105 patients—demonstrates operational execution, a key concern for biotech investors[2]. The trial's topline results, expected in Q1 2026, could catalyze a New Drug Application (NDA) submission by mid-2026[2].
The company's cash position of $35 million, sufficient to fund operations through early 2026[1], also mitigates near-term liquidity risks. This financial clarity is critical in a sector where capital constraints often derail promising pipelines. Furthermore, Quince's engagement with key opinion leaders at the 2025 A-T Clinical Research Conference and the appointment of Dr. Hassan Abolhassani to its Scientific Advisory Board signal a commitment to scientific rigor and credibility[1]. These moves likely resonate with investors prioritizing long-term value over short-term hype.
The Broader Market Context
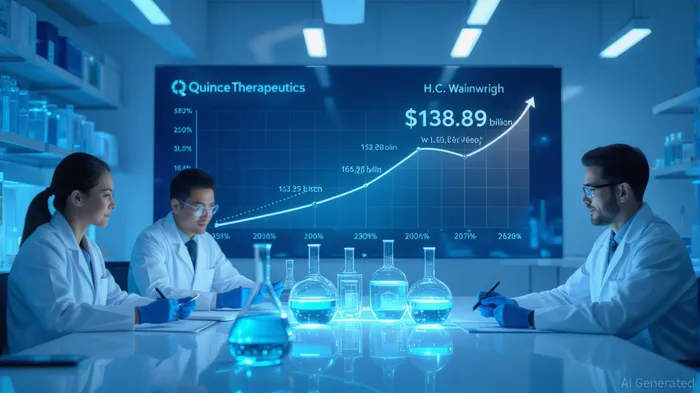
The mRNA therapeutics market, valued at $63.89 billion in 2025, is projected to grow at a 16.8% CAGR, reaching $138.88 billion by 2030[3]. Quince's focus on rare diseases—a segment with high unmet medical need and favorable regulatory pathways—positions it to benefit from this growth. Unlike competitors targeting broad applications like vaccines, Quince's niche strategy reduces direct competition while aligning with payer willingness to pay premium prices for orphan drugs.
Conclusion: A High-Stakes Bet with Clear Differentiation
Quince Therapeutics' strategic positioning in the mRNA space is defined by its ability to combine proprietary technology, rare disease focus, and operational execution. The H.C. Wainwright presentation served as a platform to highlight these strengths, reinforcing its narrative as a biotech with both scientific depth and commercial pragmatism. While the absence of immediate investor sentiment metrics limits direct assessment, the company's milestones and partnerships suggest a well-structured path to value creation. For investors, the key inflection pointIPCX-- will be the topline results from the NEAT trial in early 2026—a moment that could redefine Quince's trajectory in the mRNA therapeutics landscape.

Comentarios
Aún no hay comentarios