Novel Oncology Therapies Reshaping Small Cell Lung Cancer Treatment: Evaluating Zepzelca® and Tecentriq® Combination Therapy's Commercial Potential
The landscape of small cell lung cancer (SCLC) treatment is undergoing a transformative shift, driven by breakthroughs in immuno-oncology and targeted therapies. Among the most promising developments is the combination of Zepzelca® (lurbinectedin) and Tecentriq® (atezolizumab), which has recently secured FDA approval as a first-line maintenance therapy for extensive-stage SCLC (ES-SCLC). This approval, based on the landmark Phase III IMforte trial, underscores the therapy's potential to redefine treatment paradigms while offering significant commercial upside for stakeholders.
Clinical Efficacy and Regulatory Momentum
The IMforte trial demonstrated a 46% reduction in the risk of disease progression or death and a 27% reduction in the risk of death for patients receiving the Zepzelca-Tecentriq combination compared to Tecentriq monotherapy, according to a Genentech press release. Median progression-free survival (PFS) improved from 2.1 months with Tecentriq alone to 5.4 months with the combination, while median overall survival (OS) rose from 10.6 to 13.2 months, as reported in the FDA approval announcement. These results, presented at the 2025 ASCO Annual Meeting and published in The Lancet, led to the FDA's June 2025 approval and subsequent inclusion in NCCN guidelines, as detailed in a CURE Today article. The safety profile, though marked by increased treatment-related adverse events (e.g., nausea, anemia), remained manageable without new safety signals, per a Jazz Pharmaceuticals press release.
Market Potential and Competitive Positioning
Zepzelca's market trajectory is poised for rapid acceleration. The global Zepzelca market, valued at $199 million in 2024, is projected to reach $706 million by 2034, growing at a 13.5% CAGR, according to a market forecast. This growth is fueled by its expanding role in lung cancer and the approval of novel combinations like Zepzelca-Tecentriq. Tecentriq, already a key player in non-small cell lung cancer (NSCLC), holds a 15-20% market share in the U.S. PD-(L)1 inhibitor segment, per a pMarketResearch report. The combination therapy could further solidify Tecentriq's dominance in SCLC, a niche but high-unmet-need market.
The competitive landscape, however, is dynamic. AstraZeneca's Imfinzi (durvalumab) and emerging bispecific antibodies like Amgen's Imdelltra (amivantamab) are vying for market share. Yet, the Zepzelca-Tecentriq combo's robust clinical data-particularly its OS advantage-positions it as a preferred maintenance option, as noted in a FiercePharma analysis. Additionally, Tecentriq's subcutaneous formulation and integration into combination regimens may enhance cost-effectiveness and patient adherence compared to intravenous alternatives, a point discussed in a Synapse Patsnap article.
Pricing, Reimbursement, and Regional Dynamics
Pricing and reimbursement remain critical factors. In the U.S., high drug costs persist despite payer scrutiny, though the FDA's Priority Review and NCCN endorsement may facilitate broader coverage, as covered in a PharmExec report. Conversely, China's volume-based procurement policies have pressured prices for PD-(L)1 inhibitors, favoring domestic players like BeiGeneONC--. Multinational firms like Roche (Tecentriq's developer) and Jazz PharmaceuticalsJAZZ-- (Zepzelca's manufacturer) must navigate these disparities while emphasizing the combo's value through real-world evidence and cost-benefit analyses, supported by a Mordor Intelligence analysis.
Future Outlook and Investment Implications
The Zepzelca-Tecentriq combination represents a paradigm shift in ES-SCLC management, offering a durable, well-tolerated maintenance option. With the PDUFA date already passed and October 2025 approval secured, the therapy's commercialization is now the focus. Key risks include pricing pressures in emerging markets and competition from next-generation bispecifics. However, the combo's clinical differentiation, coupled with Zepzelca's growing market share and Tecentriq's established footprint, suggests strong long-term potential. Investors should monitor adoption rates, payer negotiations, and real-world outcomes to gauge its market penetration.
In conclusion, the Zepzelca-Tecentriq combination exemplifies how novel oncology therapies are reshaping SCLC treatment. Its regulatory success, clinical superiority, and favorable market dynamics position it as a compelling investment opportunity in the evolving immuno-oncology space.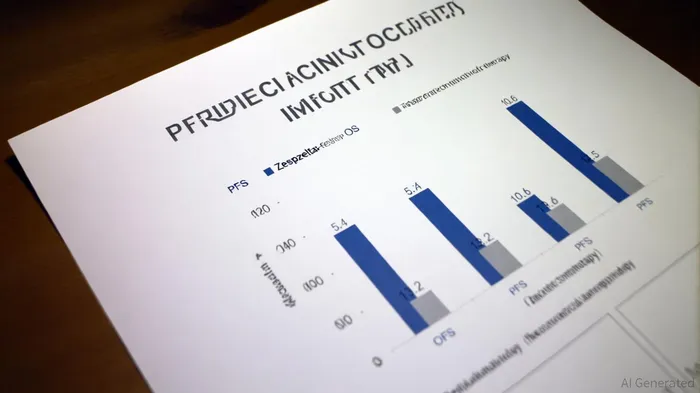

Comentarios
Aún no hay comentarios