Navigating Securities Risks in Biotech IPOs: The Imperative of Transparency and Due Diligence
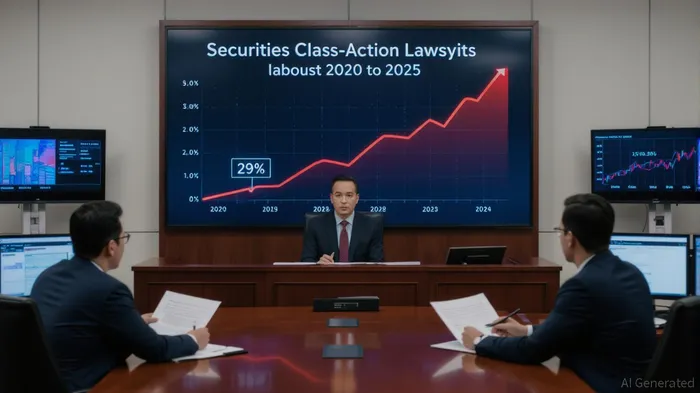
The biotech sector has long been a double-edged sword for investors: a realm of groundbreaking innovation, but also one of staggering volatility. In recent years, this duality has intensified as clinical-stage biotech firms rush to go public, only to face a deluge of securities class-action lawsuits. From 2023 to 2025, the number of such lawsuits has surged by 29%, with 44 new cases filed in 2024 alone. These legal battles often hinge on a single question: Did the company's disclosures mislead investors?
The answer, increasingly, is yes. A pattern has emerged in these lawsuits: allegations of over-optimistic clinical data, downplayed regulatory risks, and insufficient financial transparency. Consider the case of Alto NeuroscienceANRO-- (ANRO), a company that marketed its experimental drug ALTO-100 as a “first-in-class” treatment for major depressive disorder. Its 2024 IPO raised $119.6 million, buoyed by a Phase 2b trial and a memory-based biomarker strategy. When the trial failed to meet its primary endpoint in October 2024, ANRO's stock plummeted 70%, triggering a class-action lawsuit. Investors accused the company of overstating the drug's efficacy and commercial potential in its prospectus and public statements.
This case underscores a broader issue: the fragility of valuations built on speculative narratives. Clinical-stage biotech firms often rely on forward-looking statements to justify their IPO pricing, but courts are becoming increasingly skeptical. In the Ninth and Second U.S. circuits, judges now require plaintiffs to prove intentional misrepresentation—not just hindsight bias—to succeed in litigation. Yet the sheer volume of lawsuits reflects a crisis of trust. Investors are demanding accountability for companies that fail to meet expectations, and the bar for transparency is rising.
The Metrics of Transparency
For investors, the key to mitigating risk lies in evaluating a company's transparency and governance practices. Here are the critical metrics to scrutinize:
- Clinical Trial Disclosures:
- Frequency and candor in reporting trial results, adverse events, and protocol changes.
Example: Adaptimmune Therapeutics, despite a $1.14 billion accumulated deficit, has retained investor trust by rigorously updating risk disclosures and engaging with regulators.
Financial Transparency:
- Clear communication about cash runway, burn rates, and capital-raising strategies.
Red flags: Companies that obscure their financial health or rely on a single asset are more vulnerable to litigation.
Regulatory Engagement:
- Proactive disclosure of interactions with the FDA, manufacturing challenges, and feedback on drug development.
Case in point: PepGen Inc.PEPG-- faced a lawsuit after downplaying flaws in its Duchenne muscular dystrophy trial and failing to disclose regulatory concerns.
Governance Practices:
- Leadership with a proven track record in navigating regulatory hurdles and managing investor expectations.
Weak governance, as seen in ANRO, often leads to reputational and legal fallout.
Pipeline Diversification:
- Over-reliance on a single drug candidate increases risk. Firms with diversified pipelines are better positioned to weather setbacks.
Regulatory Shifts and New Challenges
The Inflation Reduction Act (IRA) of 2022 has added another layer of complexity. By allowing Medicare to negotiate drug prices, the IRA pressures biotech firms to justify valuations not just on clinical promise but on real-world pricing sustainability. This has led to a shift in investment focus from small-molecule drugs to biologics, which benefit from longer market exclusivity. However, even biologics face uncertainty under the IRA, particularly if selected for price negotiations after 11 years on the market.
The IRA's impact extends beyond pricing. It has also altered patent litigation strategies, with companies increasingly considering how to balance litigation costs against the risk of negotiated price reductions. For IPOs, this means investors must assess not only clinical and regulatory risks but also the financial implications of potential pricing caps.
Investment Advice: Balancing Hope and Caution
In this high-stakes environment, investors must adopt a disciplined approach:
- Prioritize Transparency: Favor companies that provide regular, candid updates on clinical progress and regulatory interactions.
- Demand Governance Rigor: Scrutinize leadership teams for experience in managing regulatory and financial risks.
- Leverage Legal Tools: While litigation is costly, it remains a strategic asset for holding companies accountable.
- Diversify Exposure: Avoid over-concentration in single-asset biotechs. Spread investments across firms with varied therapeutic pipelines.
The biotech sector remains a critical driver of innovation, but its risks are undeniable. As the ANRO case shows, a single misstep can trigger both a stock crash and a lawsuit. For investors, the lesson is clear: in an industry where hope and hype often collide, transparency is not just a regulatory requirement—it is a competitive advantage.
In 2025, as the sector navigates a post-pandemic correction and regulatory shifts, the companies that thrive will be those that treat transparency as a core business strategy. For investors, the path forward lies in marrying optimism with rigor—investing in innovation while demanding the accountability that markets demand.

Comentarios
Aún no hay comentarios