Natera's Strategic Momentum in Oncology Diagnostics: Market Validation and Growth Potential
Natera, Inc. (NASDAQ: NTRA) has emerged as a pivotal player in the oncology diagnostics space, leveraging its proprietary Signatera molecular residual disease (MRD) test to redefine cancer treatment paradigms. The company's strategic momentum is underscored by its robust clinical validation and expanding market applicability, as evidenced by the 14 studies presented at the 2023 European Society for Medical Oncology (ESMO) Congress. These presentations, including the landmark IMvigor011 trial in bladder cancer, position NateraNTRA-- at the forefront of personalized oncology care and highlight its potential for sustained growth.
Clinical Validation: IMvigor011 and the Bladder Cancer Breakthrough
The IMvigor011 trial, a global phase III study, represents a watershed moment for Natera's Signatera test. This trial demonstrated that circulating tumor DNA (ctDNA) monitoring can guide adjuvant immunotherapy in muscle-invasive bladder cancer (MIBC) patients. According to a Natera report, the study enrolled 761 high-risk MIBC patients post-radical cystectomy and used Signatera to detect molecular residual disease (MRD) over 21 months. Patients testing ctDNA-positive were randomized to receive atezolizumab (Tecentriq®) or a placebo, while ctDNA-negative patients were observed without treatment.
The results were striking: ctDNA-positive patients treated with atezolizumab showed statistically significant improvements in both disease-free survival (DFS) and overall survival (OS) compared to the placebo group, as reported by OncoDaily. Conversely, ctDNA-negative patients achieved excellent outcomes without additional therapy, with DFS rates of 92% at 12 months and 88% at 18 months (reported by OncoDaily). These findings validate Signatera's ability to identify patients who would benefit from adjuvant therapy while sparing others from unnecessary treatment. Natera's plans to submit a premarket approval application to the FDA for Signatera as a companion diagnostic for MIBC further underscore the trial's regulatory and commercial implications (per the Natera report).
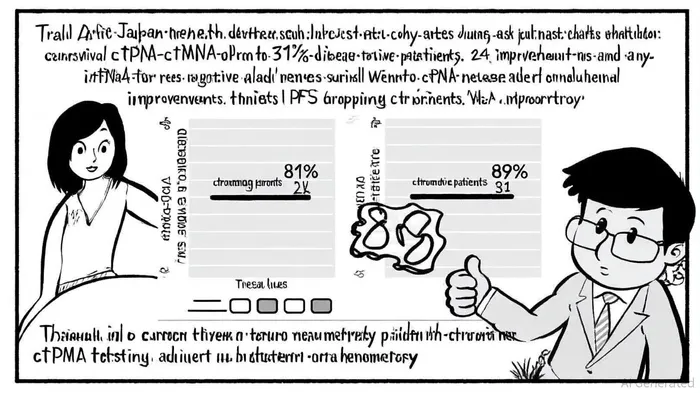
Economic and Prognostic Value in Colorectal Cancer
Beyond bladder cancer, Natera's Signatera test has demonstrated strong clinical and economic utility in colorectal cancer (CRC). Data from the CIRCULATE-Japan trial, involving over 2,625 CRC patients, revealed that ctDNA positivity was the most significant predictor of recurrence, with DFS rates plummeting from 89% to 31% at 24 months for ctDNA-positive patients. Notably, adjuvant chemotherapy (ACT) improved DFS in ctDNA-positive patients (38.6% vs. 16.1%), while no such benefit was observed in ctDNA-negative patients.
The economic impact is equally compelling. A budget impact model from BUPA indicated a 43% reduction in healthcare costs using Signatera-guided treatment versus standard of care in stage II-III CRC, a finding highlighted in the Natera report. This cost-effectiveness, combined with the test's ability to avoid overtreatment, positions Signatera as a transformative tool in oncology economics.
Broadening Applications Across Tumor Types
Natera's ESMO 2023 presentations extended Signatera's clinical relevance beyond CRC and MIBC. For instance, in rectal cancer, ctDNA monitoring predicted neoadjuvant treatment response and long-term survival outcomes. In hepatocellular carcinoma, longitudinal ctDNA assessment enabled early recurrence detection post-surgery, while in renal cell carcinoma, ctDNA monitoring predicted recurrence-free survival. These findings highlight the test's versatility and potential to disrupt treatment strategies across multiple solid tumor types.
The appendiceal adenocarcinoma study further revealed distinct mutational profiles compared to other cancers, suggesting unique oncogenic pathways and underscoring the need for tailored diagnostics. Such insights not only validate Signatera's technical capabilities but also open avenues for partnerships with pharmaceutical companies developing targeted therapies.
Market Implications and Growth Potential
Natera's strategic momentum is driven by its ability to align clinical outcomes with economic value. The IMvigor011 trial's success in bladder cancer, coupled with the CIRCULATE-Japan data in CRC, provides a robust foundation for regulatory approvals and payer reimbursement. The FDA submission for Signatera as a companion diagnostic in MIBC could unlock a new revenue stream, while the test's broad applicability across tumor types positions Natera to capture market share in multiple oncology segments.
Moreover, the growing emphasis on personalized medicine and value-based care aligns with Natera's offerings. As payers and providers prioritize cost-effective, evidence-based treatments, Signatera's ability to reduce unnecessary therapies and improve outcomes will likely drive adoption. With over 14 studies presented at ESMO 2023, Natera has effectively demonstrated its leadership in MRD testing, creating a strong narrative for long-term growth.
Conclusion
Natera's Signatera test is redefining cancer care through its precision, clinical validation, and economic benefits. The IMvigor011 trial and CIRCULATE-Japan data represent critical milestones, while the expansion into diverse tumor types underscores the test's versatility. For investors, Natera's strategic momentum-backed by robust clinical evidence and a clear path to regulatory and commercial success-positions it as a compelling opportunity in the evolving oncology diagnostics landscape.

Comentarios
Aún no hay comentarios