Nanobiotix's NBTXR3: A Game-Changer in Metastatic Melanoma and a Catalyst for Shareholder Value
In the rapidly evolving oncology landscape, NanobiotixNBTX-- (NBTX) has emerged as a compelling contender with its lead candidate, JNJ-1900 (NBTXR3), a first-in-class nanoradioenhancer. Recent Phase 1 trial results in anti-PD-1 resistant primary cutaneous melanoma underscore the therapy's potential to redefine treatment paradigms while unlocking substantial shareholder value.
Phase 1 Trial: Efficacy and Safety in a High-Need Population
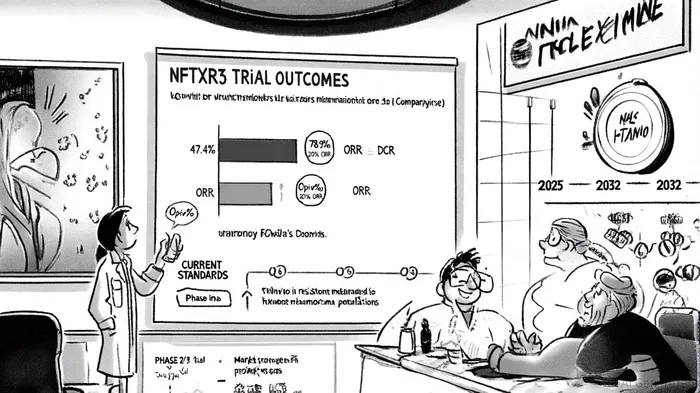
The Phase 1 study, presented at the 2025 ImmunoRad Conference, evaluated NBTXR3 in combination with immune checkpoint inhibitors for patients with primary cutaneous melanoma resistant to anti-PD-1 therapy. Among 19 evaluable patients, the best observed objective response rate (ORR) was 47.4% (9/19), including four complete responses and five partial responses, while the disease control rate (DCR) reached 78.9% (15/19). Notably, the DCR in injected and irradiated tumors was 100% (19/19), and the median overall survival (mOS) for all 21 treated patients was 14.6 months [1].
The safety profile was equally promising: only one patient experienced grade 3+ treatment-emergent adverse events (TEAEs), specifically hypotension and pleuritic pain, with no dose-limiting toxicities observed at the recommended Phase 2 dose of 33% gross tumor volume (GTV) [2]. These results suggest NBTXR3's ability to safely induce tumor cell death and re-activate immune responses, as evidenced by the correlation between local and systemic tumor regression [3].
Market Positioning: Filling a Critical Gap in Melanoma Therapeutics
The global metastatic melanoma therapeutics market, valued at USD 8.00 billion in 2025, is projected to grow at a 12.3% CAGR, reaching USD 18.02 billion by 2032 [4]. Current standards of care, such as Bristol-Myers Squibb's Opdivo and Merck's Keytruda, face limitations in resistant populations. For instance, Opdivo combined with Yervoy achieves a median overall survival (mOS) of 71.9 months but is associated with high-grade adverse events in 55% of patients [5]. NBTXR3's novel mechanism—functionalized hafnium oxide nanoparticles activated by radiotherapy—offers a scalable, one-time intratumoral injection that could overcome resistance while minimizing toxicity.
Strategic partnerships further bolster NBTX's competitive edge. A 2023 global co-development and commercialization agreement with Janssen Pharmaceutica NV provides access to J&J's infrastructure, while collaborations with The University of Texas MD Anderson Cancer Center accelerate multi-tumor-type trials [6]. Regulatory tailwinds, including the European Union's reclassification of NBTXR3 as a medicinal product (aligning with U.S. designations) and the FDA's “Study May Proceed” approval for a Phase 2 trial in non-small cell lung cancer (NSCLC), highlight its path to broad commercialization [7].
Investor Sentiment and Financials: A Strong Foundation for Growth
NBTX's stock has garnered significant attention, with a 12.9% annualized gain as of April 2025, trading at $3.24 despite a $8.00 12-month price target from analysts [8]. The company's financial runway has been extended to mid-2026 via licensing agreement amendments, reducing near-term cash burn risks. Short interest has plummeted by 68.6%, signaling growing institutional confidence [9].
The Phase 1 results, coupled with NBTXR3's versatility across solid tumors (e.g., pancreatic cancer, HNSCC), position NBTXNBTX-- to capture a substantial share of the $18B melanoma market. With four pivotal Phase 3 trials expected to redefine first-line treatment in 2025, NBTXR3's differentiation lies in its dual capacity to enhance radiotherapy and stimulate immune re-activation—a unique value proposition in an era of combination therapies [10].
Conclusion: A Catalyst for Oncology Innovation and Shareholder Returns
Nanobiotix's NBTXR3 represents a paradigm shift in metastatic melanoma treatment, addressing unmet needs in resistant populations with a safety profile that outperforms existing standards. As the company advances into Phase 2 trials and navigates a $18B market with robust partnerships and regulatory momentum, the investment case for NBTX grows increasingly compelling. For investors, the alignment of clinical innovation, market dynamics, and financial stability positions NBTX as a high-conviction opportunity in the oncology sector.

Comentarios
Aún no hay comentarios