Market-Transforming Potential of Domvanalimab + Zimberelimab + Chemotherapy in Gastroesophageal Cancer: A New Era in Immuno-Oncology
The landscape of gastroesophageal cancer treatment is on the brink of a paradigm shift, driven by groundbreaking clinical data for the combination of anti-TIGIT Domvanalimab, anti-PD-1 Zimberelimab, and chemotherapy. Recent Phase 2 results from the EDGE-Gastric trial have positioned this regimen as a potential market disruptor, offering unprecedented survival benefits and a safety profile that aligns with current standards. For investors, the implications are clear: this triple-therapy approach could redefine first-line treatment while capturing a significant share of a rapidly expanding market.
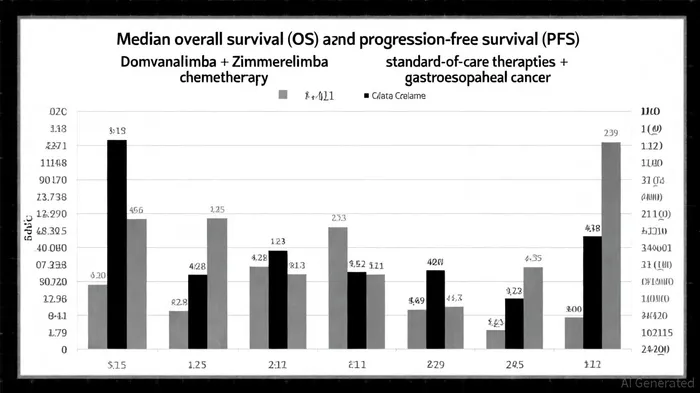
Clinical Efficacy: A Leap Beyond Current Standards
According to a report by Bloomberg, the EDGE-Gastric study demonstrated a median overall survival (OS) of 26.7 months in patients with advanced gastroesophageal adenocarcinoma treated with Domvanalimab, Zimberelimab, and chemotherapy EDGE-Gastric results. This represents a substantial improvement over existing therapies. For context, pembrolizumab combined with chemotherapy-a current standard-has shown an OS improvement of just 1.92 months over chemotherapy alone, as reported in BMC Gastroenterology BMC Gastroenterology. The 26.7-month median OS in the Domvanalimab/Zimberelimab arm translates to a 50.2% 24-month survival rate, a metric that could redefine expectations for this aggressive disease.
The regimen's efficacy spans all PD-L1 subgroups, a critical advantage in a disease where biomarker heterogeneity often limits treatment options. Additionally, the 12.9-month median progression-free survival (PFS) observed in the trial outperforms the 7.3-month PFS reported for camrelizumab-based combinations, further underscoring its competitive edge.
Market Context: A $36.62 Billion Opportunity by 2035
The global gastroesophageal cancer treatment market is projected to grow at a 8.23% CAGR, reaching $36.62 billion by 2035, according to a GlobeNewswire projection GlobeNewswire projection. This growth is fueled by unmet needs such as late-stage diagnosis, treatment resistance, and the high toxicity of existing regimens. Domvanalimab + Zimberelimab + chemotherapy addresses these gaps by combining immune checkpoint inhibition with chemotherapy, a strategy that enhances tumor cell death while minimizing off-target effects.
Notably, the STAR-221 Phase 3 trial-directly comparing this regimen to nivolumab + chemotherapy-is poised to validate these findings in a larger cohort. If successful, the therapy could secure a first-line label, capturing a significant portion of the $15.34 billion 2024 market referenced in the same GlobeNewswire analysis.
Competitive Positioning: Safety and Efficacy Synergy
Safety remains a critical differentiator. While pembrolizumab monotherapy reduces adverse event (AE) risk compared to chemotherapy, the Domvanalimab/Zimberelimab combination has shown no unexpected safety signals, with treatment-emergent AEs (TEAEs) primarily attributable to chemotherapy. This aligns with investor expectations for therapies that balance efficacy with tolerability-a key factor in adoption by oncologists and payers.
Moreover, the dual inhibition of TIGIT and PD-1 pathways represents a novel mechanism. By blocking both checkpoints, the regimen amplifies T-cell activation and macrophage engagement, a strategy that competitors like AstraZenecaAZN-- and Roche are racing to replicate but have yet to match in clinical outcomes.
Future Outlook: A Pipeline of Potential
The STAR-121 trial in non-small cell lung cancer (NSCLC) further validates the versatility of this combination, with 720 patients enrolled to compare Domvanalimab/Zimberelimab + chemotherapy against pembrolizumab + chemotherapy, as listed on PubMed PubMed listing. Positive results here could expand the therapy's label beyond gastroesophageal cancer, unlocking additional revenue streams.
For investors, the path forward is clear: Domvanalimab + Zimberelimab + chemotherapy is not just a treatment-it's a market-transforming force. With Phase 3 trials underway and a $36.62 billion market on the horizon, this regimen could become the new gold standard, redefining survival metrics and investor returns alike.

Comentarios
Aún no hay comentarios