Lilly's Mounjaro and the Pediatric Diabetes Breakthrough: A Catalyst for Long-Term Growth in a Booming Market
The global Type 2 diabetes treatment market is undergoing a seismic shift, driven by rising obesity rates and the alarming rise of diabetes in younger populations. At the forefront of this transformation is Eli Lilly's Mounjaro (tirzepatide), a dual GIP/GLP-1 receptor agonist that has demonstrated groundbreaking efficacy in pediatric trials. With regulatory submissions underway and a robust clinical profile, Mounjaro's potential expansion into the pediatric market could redefine Lilly's growth trajectory in a sector projected to exceed $100 billion by 2030[1].
Clinical Breakthrough: Efficacy and Safety in Pediatric Patients
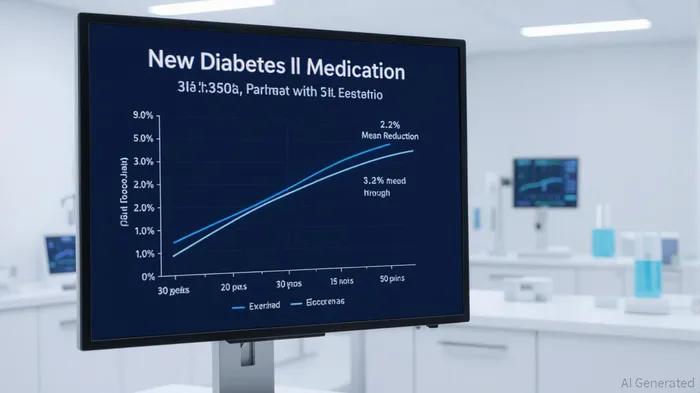
According to a report by Bloomberg, Eli Lilly's Phase 3 SURPASS-PEDS trial revealed that Mounjaro reduced A1C levels by 2.2% in children and adolescents aged 10–17 with Type 2 diabetes, compared to a 0.05% increase in the placebo group[2]. This translates to 86.1% of participants on the 10 mg dose achieving the target A1C level of ≤6.5%, a critical benchmark for diabetes management[2]. Additionally, the drug drove an 11.2% reduction in BMI at 30 weeks, with sustained weight loss through a 52-week extension period—a rarity in diabetes therapies[3].
The safety profile, consistent with adult trials, included mild-to-moderate gastrointestinal side effects, with no severe hypoglycemia reported[4]. As noted by Reuters, these findings position Mounjaro as a viable option for a demographic historically underserved by diabetes treatments[5].
Regulatory Momentum and Market Expansion
Lilly has submitted the trial data to global regulatory agencies, signaling its intent to secure pediatric approval[6]. While the drug remains unapproved for patients under 18 as of Q3 2025, the strong clinical results and the urgent unmet need in pediatric diabetes care suggest a high likelihood of regulatory clearance within 12–18 months. This timeline aligns with the growing emphasis on obesity and diabetes management in youth, a trend amplified by the World Health Organization's 2025 Global Obesity Strategy[7].
Competitive Edge: Dual Agonism and Long-Term Efficacy
Mounjaro's dual GIP/GLP-1 mechanism offers a distinct advantage over single-agonist therapies like Novo Nordisk's Wegovy and Ozempic. Data from the SURPASS-PEDS trial underscores its ability to maintain weight loss without plateauing—a critical factor for long-term adherence[8]. In a market where GLP-1 drugs dominate, Mounjaro's dual-action approach could capture a significant share, particularly in pediatric populations where safety and efficacy are paramount[9].
Long-Term Growth: A Strategic Play on an Expanding Market
The pediatric diabetes segment is a $12 billion niche today but is expected to grow at a 15% CAGR through 2030, driven by rising obesity rates and earlier disease onset[10]. Lilly's early mover advantage, combined with its pipeline of trials targeting children as young as six years old[11], positions the company to dominate this emerging market. Furthermore, Mounjaro's potential approval could unlock cross-selling opportunities with Lilly's broader diabetes portfolio, including insulin therapies and the weight management drug Zepbound.
Conclusion
Eli Lilly's Mounjaro represents more than a therapeutic innovation—it is a strategic lever for capturing a rapidly expanding market. With compelling clinical data, regulatory momentum, and a unique dual-agonist mechanism, the drug is poised to redefine pediatric diabetes care while driving long-term revenue growth. For investors, the pending approval and Lilly's aggressive R&D pipeline in younger demographics present a compelling case for sustained value creation.

Comentarios
Aún no hay comentarios