Johnson & Johnson's FDA-Approved INLEXZO for Bladder Cancer and Its Market Potential
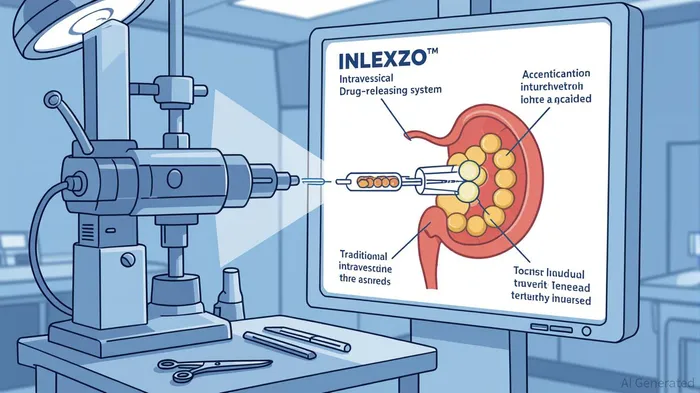
Johnson & Johnson's recent FDA approval of INLEXZO™ (gemcitabine intravesical system) marks a transformative milestone in uro-oncology, addressing a critical unmet need in the treatment of high-risk non-muscle invasive bladder cancer (NMIBC). This novel intravesical drug-releasing system (iDRS) is the first and only therapy designed to deliver sustained local chemotherapy over three weeks per treatment cycle, offering a bladder-preserving alternative to radical cystectomy for patients unresponsive to Bacillus Calmette-Guérin (BCG) therapy [1]. With clinical data demonstrating an 82% complete response rate and 51% durability of response at one year, INLEXZO™ has redefined the therapeutic landscape for a patient population with limited options [2]. For JohnsonJNJ-- & Johnson, the drug represents not just a medical breakthrough but a strategic pillar in its ambition to dominate the growing bladder cancer market and achieve $50 billion in oncology sales by 2030 [3].
A Paradigm Shift in Bladder Cancer Treatment
Bladder cancer remains a significant global health burden, with over 570,000 new cases diagnosed annually. Non-muscle invasive bladder cancer (NMIBC), which accounts for ~70% of cases, is typically managed with intravesical therapies or surgical interventions. However, patients with BCG-unresponsive NMIBC—particularly those with carcinoma in situ (CIS)—face a grim prognosis, as current treatments often fail to prevent disease progression or recurrence [4]. Radical cystectomy, while effective, is a life-altering procedure with substantial morbidity, making bladder-sparing alternatives a priority for both patients and clinicians.
INLEXZO™'s mechanism of action addresses this gap. The device, a flexible, pretzel-shaped polymer matrix, is inserted via catheter and remains in the bladder for three weeks, continuously releasing gemcitabine. This prolonged exposure enhances drug efficacy while minimizing systemic toxicity, a key limitation of traditional intravesical therapies [5]. Clinical data from the Phase 2b SunRISe-1 trial underscores its potential: 82.4% of patients achieved complete response (CR), with 52.9% maintaining CR for at least one year [6]. These results, coupled with the therapy's outpatient administration and absence of general anesthesia requirements, position INLEXZO™ as a practice-changing innovation [7].
Market Potential and Competitive Landscape
The bladder cancer treatment market, valued at $5.11 billion in 2024, is projected to grow at a compound annual growth rate (CAGR) of 5.4% through 2033, reaching $8.15 billion [8]. This expansion is driven by advancements in immunotherapy, targeted therapies, and innovations like INLEXZO™ that address unmet needs. Johnson & Johnson's entry into this space is particularly strategic, as it leverages its expertise in drug-device combinations—a domain where it has historically excelled.
Competitive analysis reveals a fragmented market dominated by systemic chemotherapies (e.g., cisplatin) and immunotherapies (e.g., checkpoint inhibitors), but few localized solutions for BCG-unresponsive NMIBC. While companies like UroGen PharmaURGN-- and Tyra BiosciencesTYRA-- are developing novel intravesical agents, INLEXZO™'s first-mover advantage and FDA approval provide J&J with a significant edge. According to Bloomberg, the company projects peak annual sales of INLEXZO™ to exceed $5 billion, a figure three times higher than Wall Street estimates, reflecting its confidence in market adoption [9].
Strategic Implications for Johnson & Johnson's Oncology Division
INLEXZO™ aligns with Johnson & Johnson's broader oncology strategy, which emphasizes high-growth therapeutic areas and innovative platforms. The company's oncology segment reported 24% year-over-year revenue growth in Q2 2025, driven by products like Darzalex, Erleada, and Carvykti, as well as emerging therapies like INLEXZO™ [10]. With bladder cancer representing a $5.7 billion market by 2035 (CAGR: 3.29%), J&J's focus on this niche strengthens its pipeline diversification and long-term revenue stability [11].
Moreover, INLEXZO™'s success could catalyze further innovation in localized drug delivery systems, a domain where J&J has invested heavily. The company's experience with devices like its Synchro™ catheter system and its expertise in polymer-based drug release technologies position it to iterate on INLEXZO™'s platform, potentially expanding its use to other urological cancers or indications.
Risks and Considerations
Despite its promise, INLEXZO™ faces challenges. Adverse events such as urinary frequency, dysuria, and bladder irritation, though generally mild, could impact patient adherence [12]. Additionally, reimbursement hurdles and competition from generic chemotherapies may constrain margins. However, J&J's robust commercial infrastructure and partnerships with urology KOLs mitigate these risks.
Conclusion
Johnson & Johnson's INLEXZO™ is poised to redefine bladder cancer care, offering a durable, bladder-sparing solution for a high-risk patient population. With its groundbreaking mechanism, strong clinical data, and strategic alignment with J&J's oncology ambitions, the therapy represents a $5 billion+ opportunity. As the bladder cancer market expands, INLEXZO™ not only addresses an urgent medical need but also cements Johnson & Johnson's leadership in uro-oncology—a critical component of its $50 billion oncology sales target by 2030.

Comentarios
Aún no hay comentarios